The Medical Device Authority (MDA) of Malaysia has published a guidance document dedicated to the requirements for registration of conformity assessment bodies (CABs). In particular, the document describes the requirements the entity shall meet in order to be eligible for conducting conformity assessment of medical devices.

Regulatory Background
The present MDA guidance is based on the current Malaysian legislation on medical devices, which includes, inter alia, the following acts and regulations:
- Medical Device Act 2012 (Act 737),
- Medical Device Regulations 2012,
- Circular Letter of the MDA No.2 / 2014 on Conformity Assessment by way of Verification.
- ISO 13485, Medical Device – Quality Management – Systems – Requirements for regulatory purposes.
In case of any contradictions between the aforementioned laws and regulations and the present guidance, the provisions set forth by the laws and regulations shall prevail.
However, the authority reserves the right to set forth any additional requirements it will deem necessary to ensure the safety and quality of medical devices allowed to be marketed in Malaysia. The MDA is also entitled to amend the guidance.
In accordance with the Fourth Schedule of the Medical Device Regulations (MDR) 2012, any conformity assessment body should be duly registered in order to be allowed to perform conformity assessment under the Third Schedule of the aforementioned Regulation.
First of all, the MDA provides the definitions of the most important terms to be used in the context of conformity assessment, namely:
- Guide – person appointed by the client to assist the audit team,
- Impartiality – presence of objectivity (no conflict of interest shall exist, or it should be resolved in a way insuring it would not impact the certification activities),
- Management system consultancy – participation in establishing, implementing, or maintaining a management system,
- Observer – person who accompanies the audit team but does not perform the audit,
- Technical expert – CAB appointed expert who possess relevant qualification and experience to support specific technology areas in the conduct of specific medical device conformity assessment,
- Technical personnel – CAB personnel who possess relevant qualifications and experience to conduct medical device conformity assessment on technical documentation and Declaration of Conformity based on his/her areas of expertise in Appendix 1 of Fourth Schedule of MDR 2012.

Organizational Requirements for CABs in Malaysia
The MDA guidance also provides detailed requirements an entity shall meet in order to be eligible for designation as a conformity assessment body in Malaysia. When applying for registration as a CAB, the entity shall consider the following points:
- The person responsible for the management and operations shall be a Malaysian citizen,
- In case if the applicant entity is a subsidiary of a larger structure, the application be accompanied with the detailed information about the parent company and whole structure in general,
- The way the certification activities are structured and managed shall ensure the impartiality,
- The entity shall have a clearly identified management structure,
- All internal bodies conducting certification activities should be formed and managed in accordance with the appropriate internal rules,
- The CAB will be fully responsible for all activities it performs and for all decision to be made in the course of such activities,
- When making a decision on certification, the conformity assessment body shall duly assess all the evidence,
- In case of any significant changes to its internal structure, the management, or other important aspects, the conformity assessment body shall duly inform the regulating authority within 14 days from the date such changes actually took place.
Additional Requirements for Conformity Assessment Bodies
Besides the organizational criteria described hereabove, the MDA guidance also outlines special requirements on resources and technical competency the applicant entity shall meet. In particular, the entity applying for the registration as a conformity assessment body shall employ the personnel having the necessary qualification and competence, as well as facilities and testing equipment necessary to conduct conformity assessment activities. The CAB shall also have at least one lead auditor being a full-time employee.
Moreover, the auditors and technical personnel of the conformity assessment body should be registered with the MDA. The CAB shall also develop internal rules regulating the procedure to be applied when selecting and appointing the audit team consisting of the technical experts and the audit team lead. Such a selection should be based on the competencies reasonably necessary due to the scope of the planned audit.
The guidance also describes the qualification requirements the CAB personnel shall meet. The whole information could be provided in a form of a table.
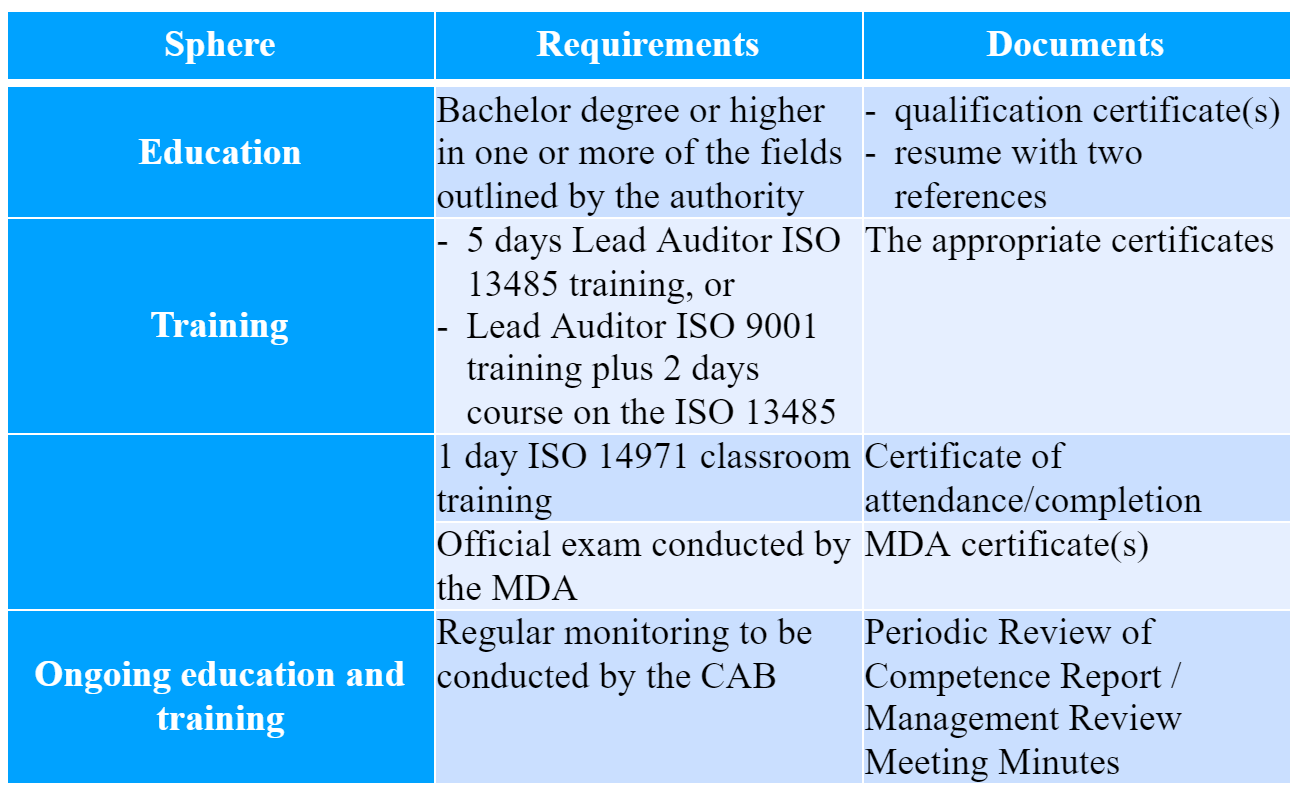
According to the MDA guidance, the qualification and performance of the auditors should be assessed by the conformity assessment body on the annual basis. The CAB shall duly perform the aforementioned activity and provide the appropriate documents, otherwise, the registration of personnel will be suspended. Thus, the CAB shall demonstrate the fulfillment of the obligations on maintaining and monitoring the qualification and competence of the personnel.
Work Experience Requirements
Another important aspect covered by the MDA guidance on requirements for conformity assessment bodies relates to the work experience of the auditor and technical personnel. In particular, the following requirements should be applied:
- The work experience of the auditor should include, in general, at least 20 audits and cover the review of documentation and risk management of medical devices plus the audits performed in four different organizations as a trainee auditor.
- The work experience of audit team leaders should also include an experience of participation in audits in the appropriate leading role while being supervised by a qualified team leader during at least three audits.
- Technical personnel shall have at least four years of full-time experience in the industry, two of them in the spheres close to research and development, manufacturing, healthcare services, testing medical devices, etc. The number of years of experience required to meet the eligibility criteria in case of Master of Ph.D. degree – for one and three years respectively.
- The technical expert should have at least eight years of experience in the relevant sphere, together with the appropriate degree.
The initial CAB registration remains valid for 3 years. In order to be allowed to conduct conformity assessment, the CAB shall submit a new application for registration at least one year prior to the expiration of the initial registration period. According to the registration requirements, the conformity assessment body is obliged to maintain the competence of its auditors and technical personnel within the whole period of registration to be eligible for the registration for an additional term.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:

