Easily track and report on global medical device registrations
Eliminate Costly Mistakes and Missed Deadlines
Often medical device companies rely on homegrown spreadsheets and disparate systems to track their global registrations. This creates opportunities for costly mistakes that can result in lost revenue, resources, and even products being removed from the market.
With RegDesk, you can eliminate the risk of human error to safeguard and streamline the entire product lifecycle.
RegDesk's built-in reporting and statistics in their Tracking module allows our regulatory leader to easily present the KPIs to the organization. We are also able to keep track of the incoming international requests which allowed for our company to enhance its approach on global expansion.
Olivia P., Senior Director, Regulatory Affairs
Large Medical Device Company
With RegDesk's Medical Device Inventory Tracking Software, You Can:
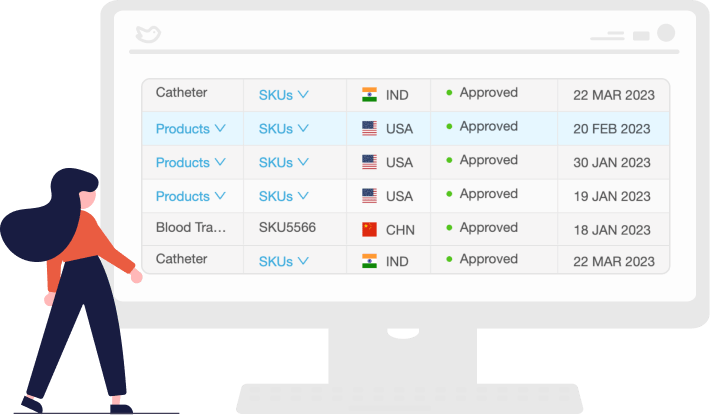
Track the Status of Registrations Globally
See the status of registrations, simplify the tracking of regulatory approvals, and stay ahead of regulatory changes to remain compliant and avoid costly penalties.
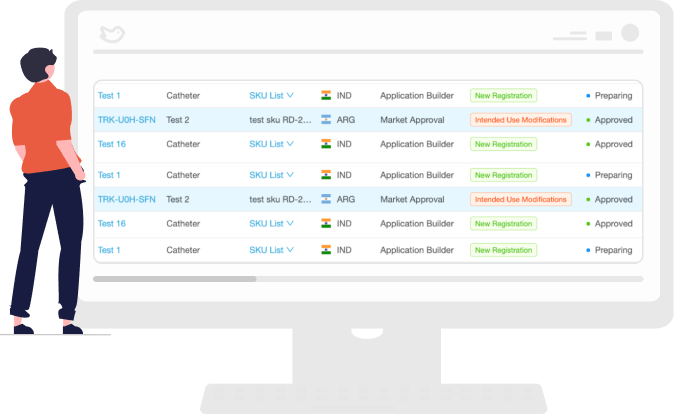
Receive Renewal Notifications
View the regulatory landscape for your product and be notified of renewal requirements from various regulatory bodies and easily identify documents that need to be resubmitted or updated.
Then you can prioritize your regulatory obligations to remain compliant and easily manage the renewal process.
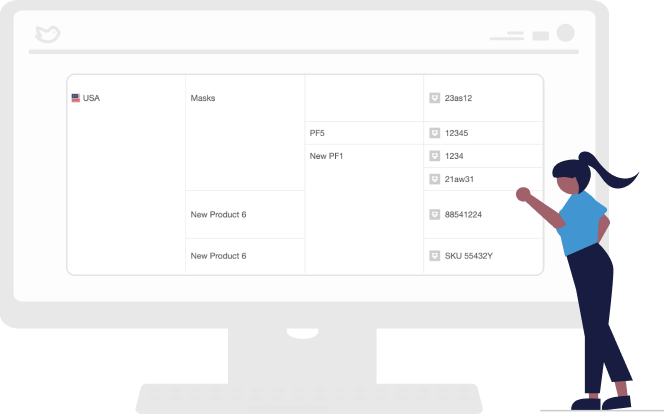
Report on KPIs In Seconds
Generate accurate and timely performance reports with ease. RegDesk’s advanced algorithms quickly search through large amounts of data to generate comprehensive reports with detailed insights and analysis on changes, trends and issues so you can make informed compliance decisions.
Report on KPIs In Seconds
Generate accurate and timely performance reports with ease. RegDesk’s advanced algorithms quickly search through large amounts of data to generate comprehensive reports with detailed insights and analysis on changes, trends and issues so you can make informed compliance decisions.

Who Can Use Medical Tracking Device Software
A digitized automated medical tracking system is a necessity for regulatory teams that are:

Using spreadsheets to track global registrations

Spending hours fiddling with data to generate key reports using Excel

Struggling to monitor the status of global registrations

Missing deadlines on renewals

Shipping unapproved products to the wrong countries
