Stay current with ever-changing global standards
All-in-One Medical Device Standards Management
The obstacles to launching a medical device can be daunting. Compliance with international and country-specific standards is paramount to success. However, those standards are constantly changing.
With RegDesk, you can be alerted to these changes, perform a gap analysis of new and revised standards, and understand how these changes impact your portfolio and submissions.
We love RegDesk's Standard Management product. We have all the data required for an audit, including gap assessments set up in the system.
Jacelyn B. Associate Director RA,
Large MedTech Company
With RegDesk's Medical Device Standards Management, You Can:
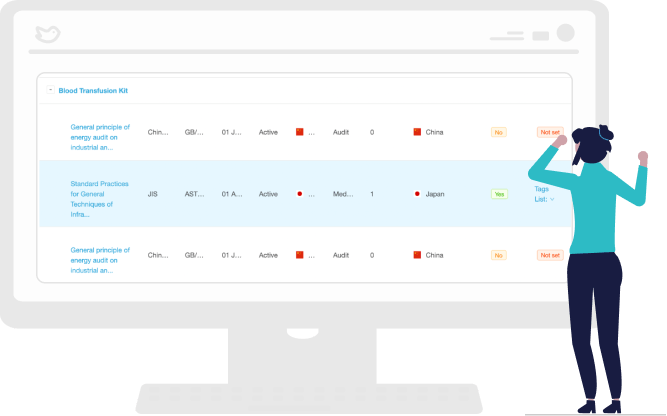
Manage International and Country-Specific Standards by Product
Stay on top of ever-evolving standards with a centralized repository of product-specific standards so you can remain globally compliant.
The RegDesk comprehensive solution helps you quickly access relevant information applicable to your products and provides a dashboard to identify trends and assess performance against global and country-specific standards.
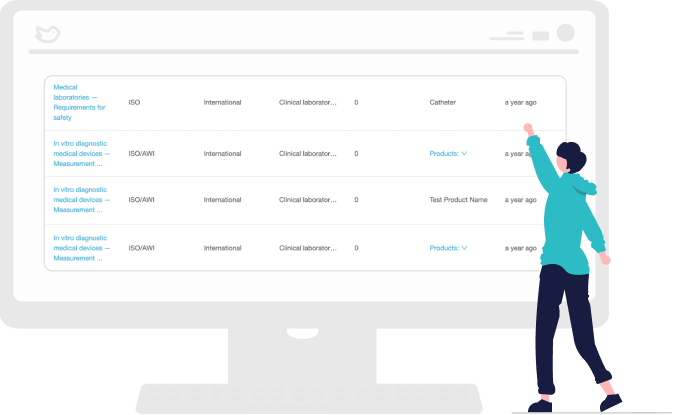
Receive Alerts on Changing Standards
RegDesk advanced algorithms monitor changes to standards in real-time. Monthly alerts notify users of changes in standards and empower teams to summarize and document how those changes may impact products. This helps you stay ahead of changes and remain compliant.
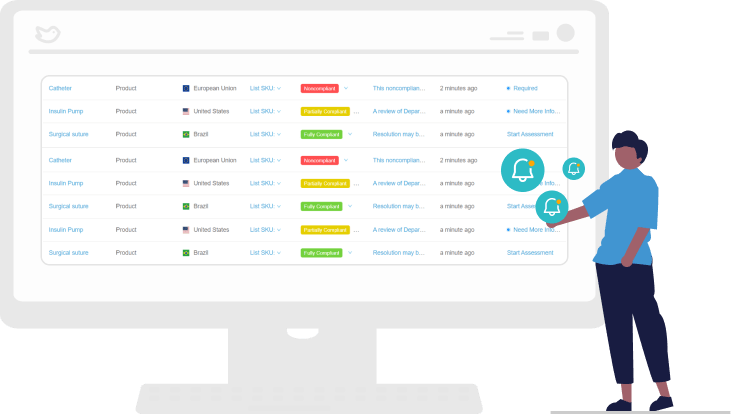
Perform Gap Assessments
Automate your workflow so corporate and business units can perform individualized gap assessments and share their findings. This makes it easier for external auditors to trace the change assessment to standards and reduces the risk of non-compliance.
Perform Gap Assessments
Automate your workflow so corporate and business units can perform individualized gap assessments and share their findings. This makes it easier for external auditors to trace the change assessment to standards and reduces the risk of non-compliance.

Do You Need a Standards Management Solution?
Regulatory teams should consider a standards management tool if they are:

Trying to eliminate non-compliance notices from health agencies and save hundreds of thousands in unnecessary product testing.

Struggling to keep up with ever-changing standards globally.

Facing increased exposure to compliance vulnerabilities.

Using manual, paper-based methods to document gap assessments.
Manually working from a list of medical device standards to conduct a gap analysis is overwhelming and time-consuming. Streamline the process with the right software by your side.
