Trusted By
Stay ahead of global change with a regulatory compliance solution
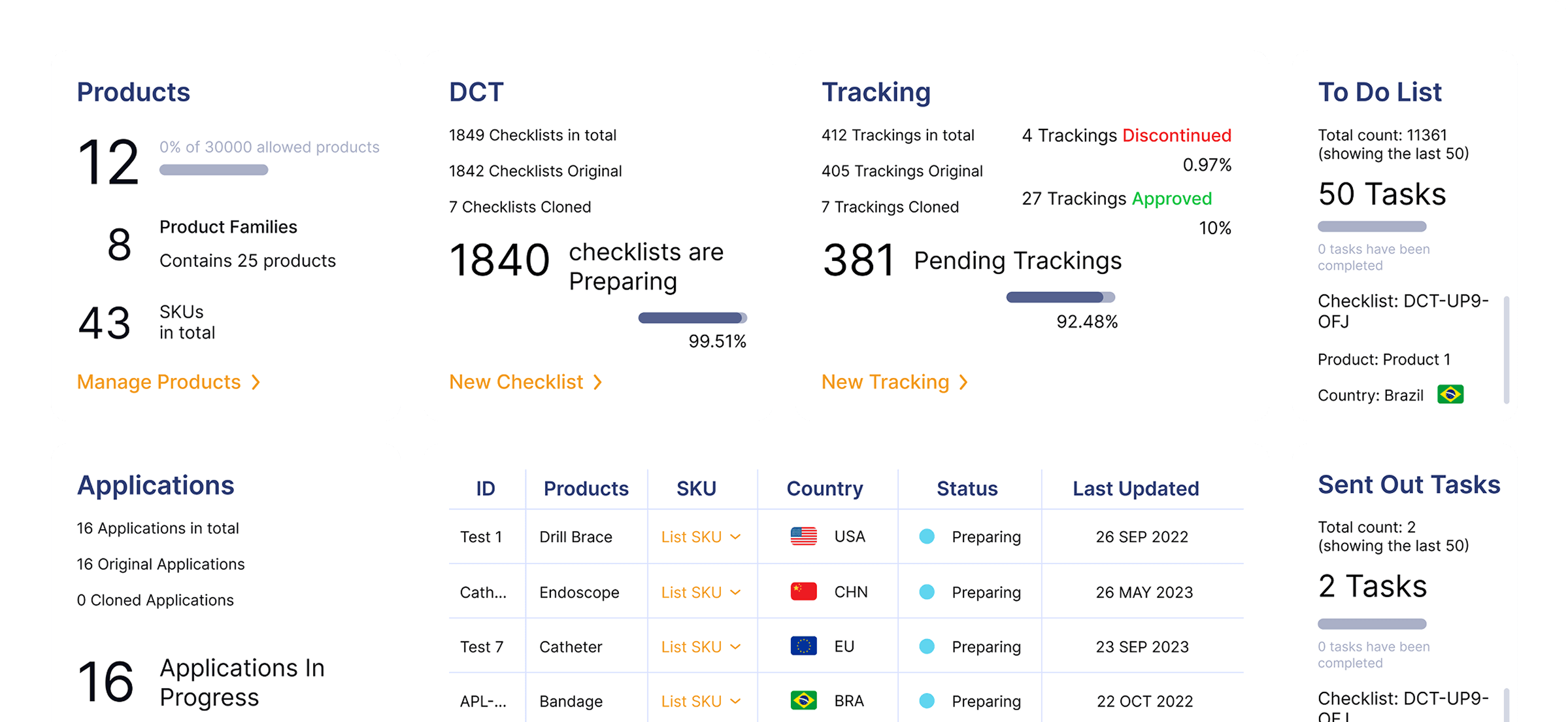
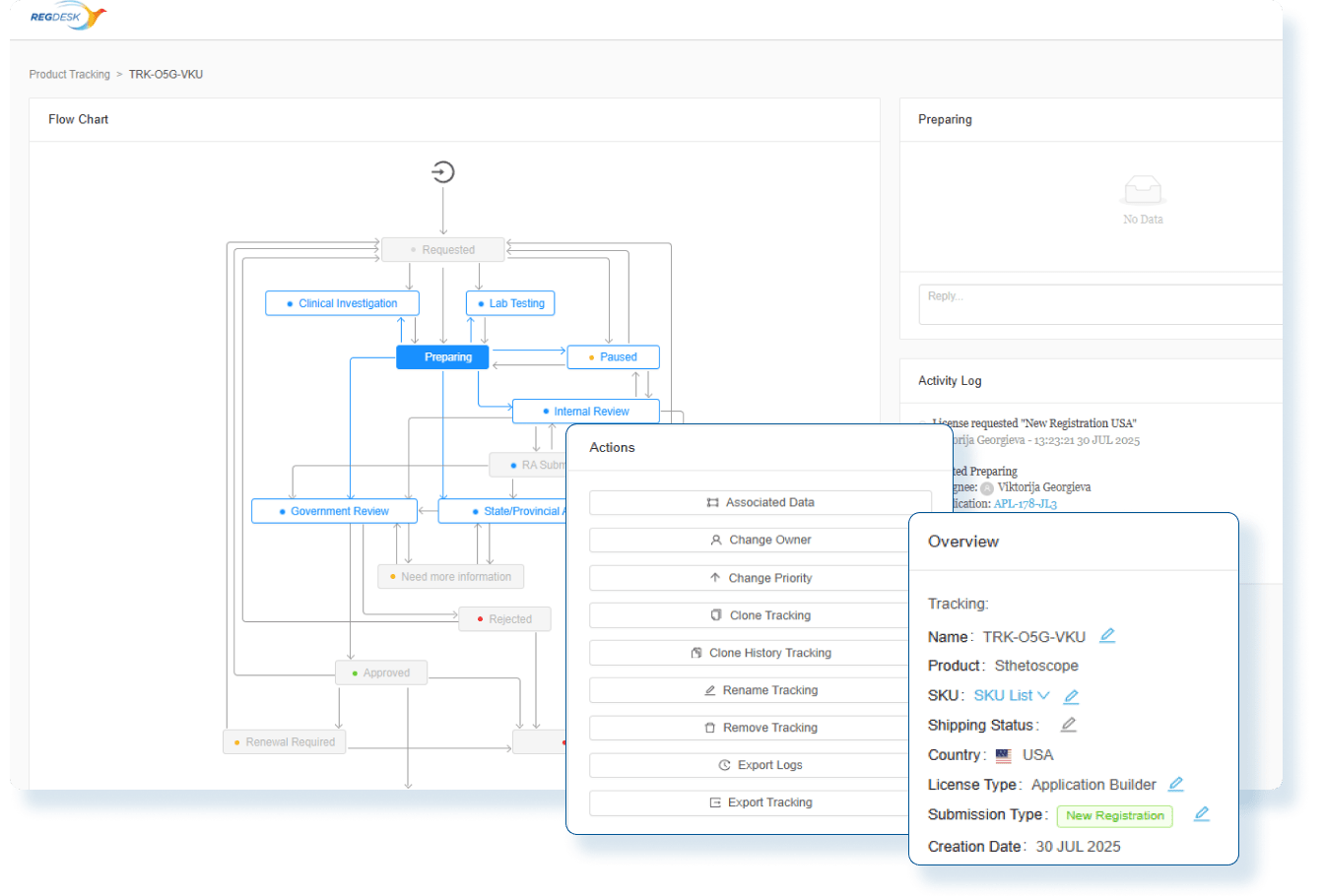
How RIMS WorksAn all in one solution that will solve your regulatory challenges by streamlining submissions and enabling smarter workflows to save time and eliminate repetitive tasks. Regdesk provides a simple path for regulatory strategic development with real-time regulatory updates and simplified tracking and reporting so medical device manufacturers can eliminate compliance risks and avoid costly penalties. Empower your team to adapt quickly and comply with regulatory changes with confidence.
Regulatory Compliance Platform
A one-stop Regulatory Compliance Platform with an end-to-end solution for regulatory teams.
Real Time Visibility
Gain insights into global submissions and generate KPI reports to enhance global submission.
Data-driven Decisions
Make smarter, faster decisions with customizable analytics
Improved Accountability
Enhance cross-functional alignment and collaboration with audit-ready tracking and reporting.
“Our regulatory affairs team is much more efficient and we are better able to measure and track our KPIs.”
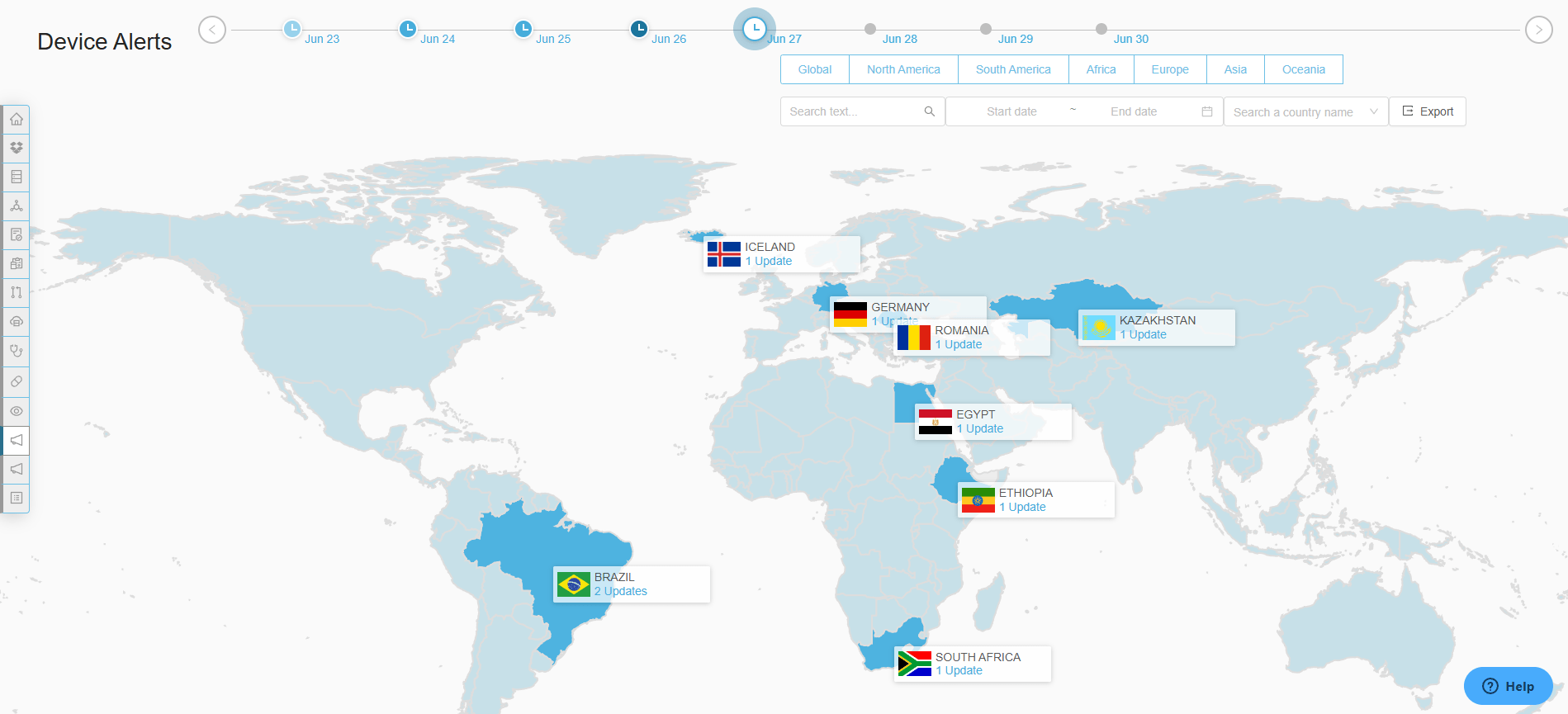
Proactive Compliance
Stay ahead of evolving regulations with real-time alerts and updates.
Strategic Oversight
Gain a global perspective on regulatory changes to manage resources, plan launches, and support long-term growth initiatives.
Faster Market Access
Accelerate decision making with country specific regulatory requirements.
Enhanced Efficiency and Productivity
Stay ahead of the ever-evolving regulatory landscape with real-time regulatory updates on changes to regulations.
“Regulatory Intelligence is always difficult so having a module that is seamless, updated and always ready allows you to stay competitive.”
– Elizabeth H. at Zoll
Accelerated Time to Market
Prepare submission applications in days vs months with comprehensive country specific medical device registration templates.
Operational Efficiency
Eliminate rework and errors while freeing up regulatory teams to focus on strategic initiatives
Scalable Global Processes
Confidently support expansion into new markets with high-quality forms and submissions without adding headcount or external resources.
“RegDesk saved us 11 months of time. We will definitely be using them again.”
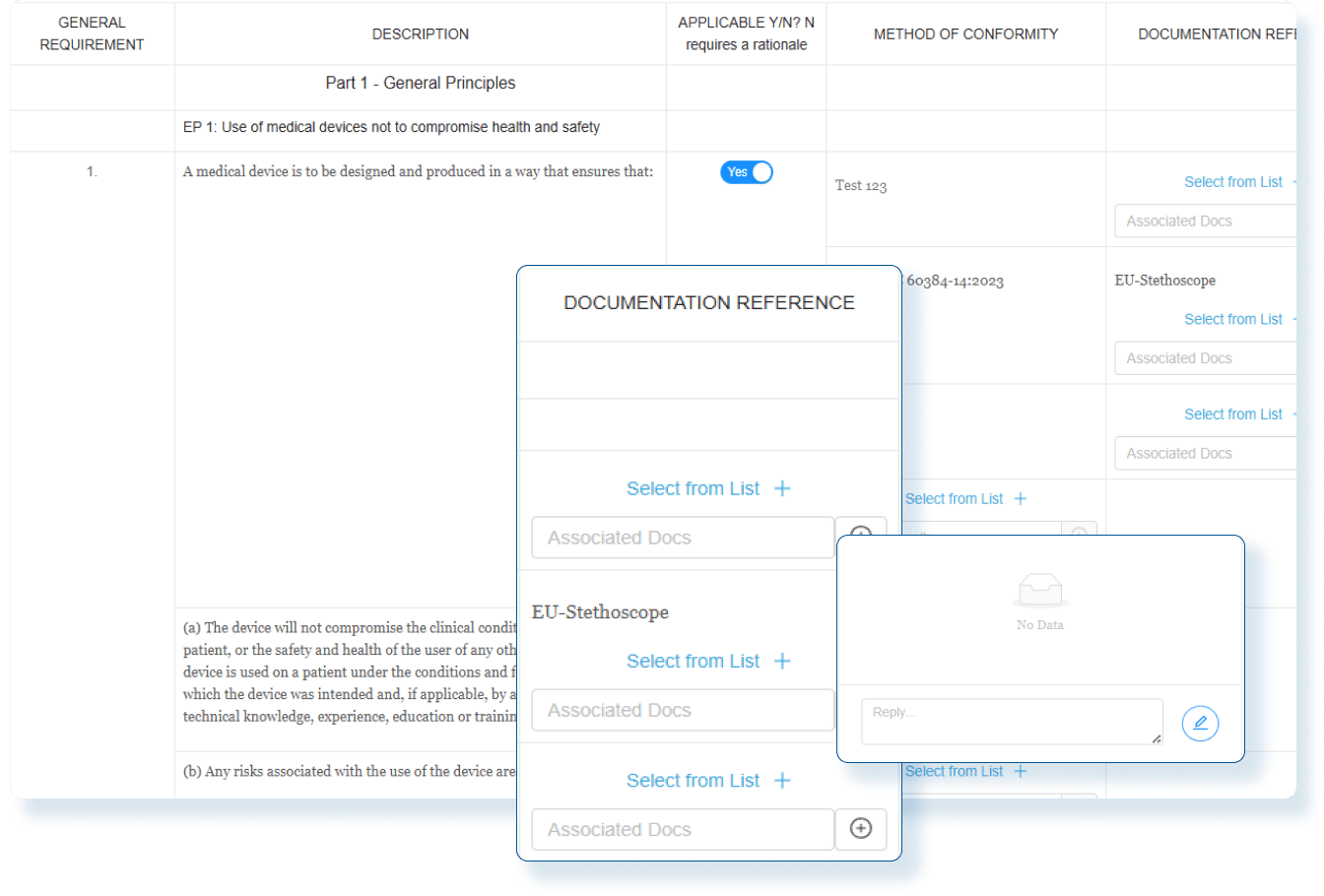
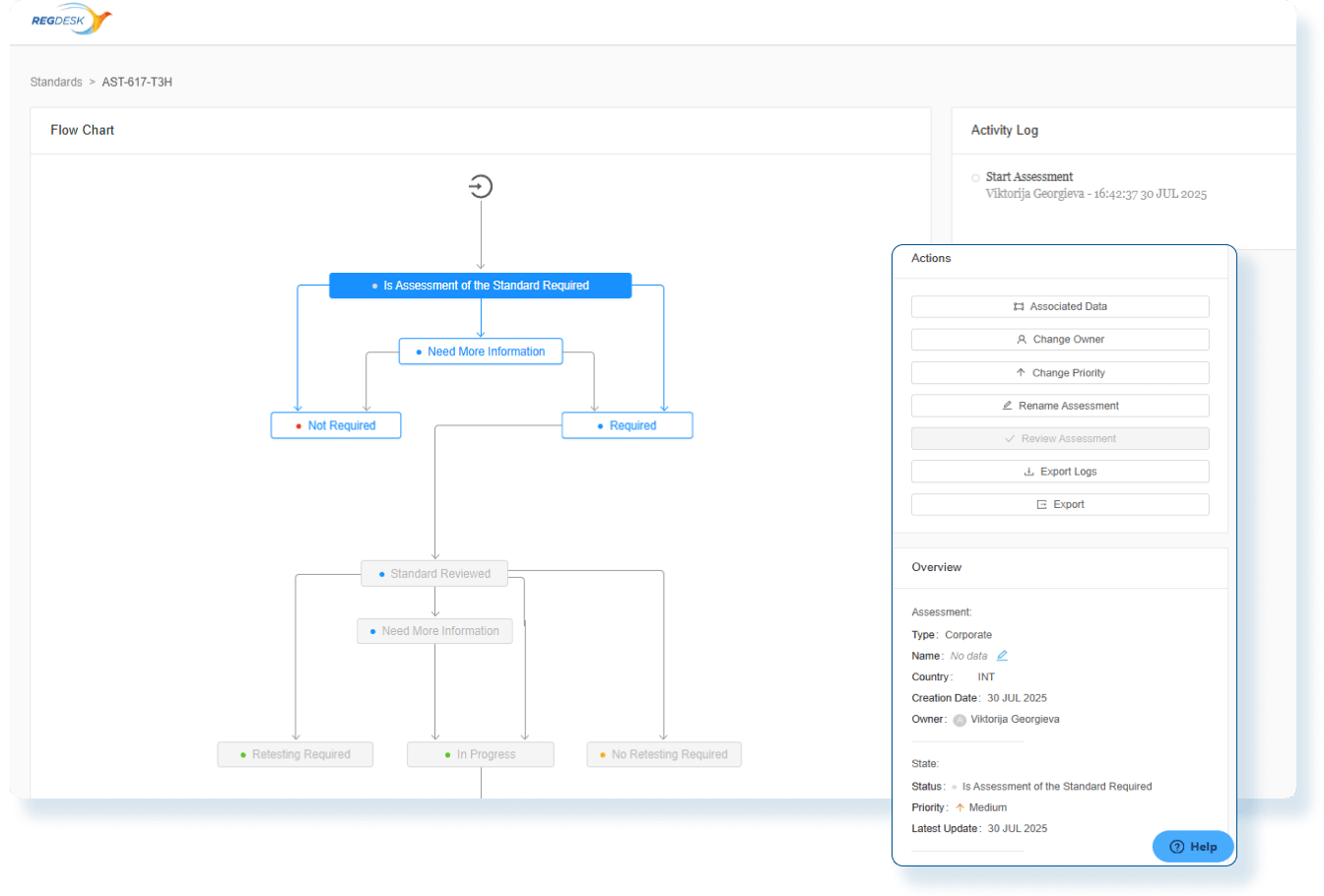
Assess Global Change Impact
Instantly evaluate the global impact of every product change and produce smarter regulatory decisions with clear, actionable insights.
Evaluate Compliance Risks Before Launch
Use comprehensive assessments with decision-tree workflows so Regulatory Affairs team clearly understand the global regulatory impact before market release.
Stay Audit Ready and Globally Compliant
Every change is backed by impact assessments and documented actions so you can confidently make changes and launch updated products in any market.
“Compliance tracking allows companies to establish and maintain a validated state of control, while looking at efficient ways to stay ahead of changing regulations.”
The Proof is in the Numbers
The Total Economic Impact™
Forrester TEI study finds 196% ROI with RegDesk
Market Served
Savings on Product Evaluation Costs and Timelines
Products Registered
Through RegDesk
Reduction in
Regulatory Risk
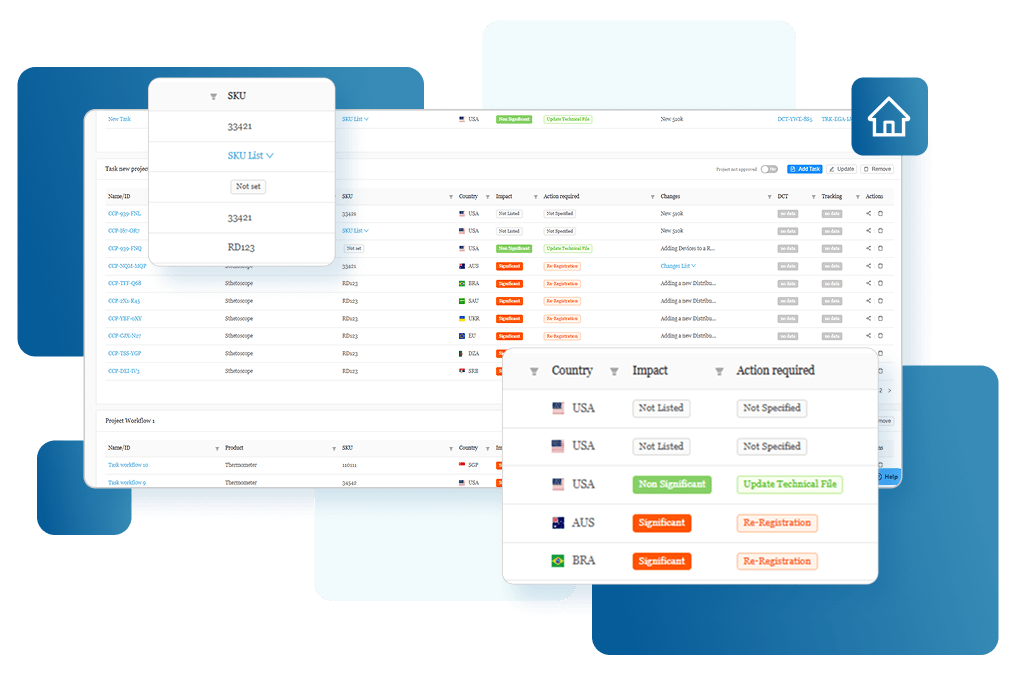
Streamline Compliance with RegDesk
Regulatory Compliance Management Has Never
Been Simpler
Streamline your complex processes, boost operational efficiency, and minimize manual tasks. Foster collaboration, reduce errors, enhance inter-departmental communication, and make your regulatory strategy more effective and manageable.
Request a Demo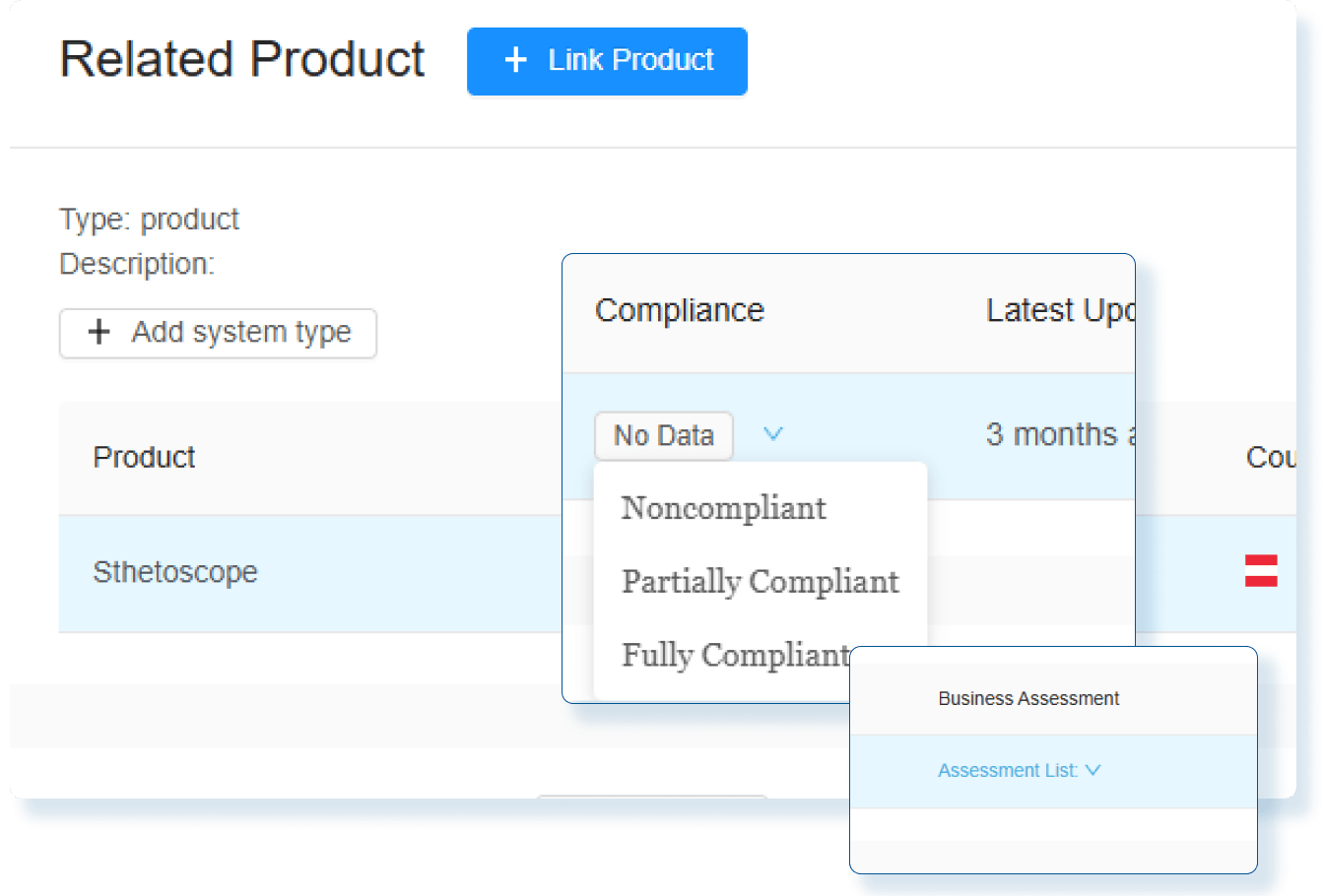
Proactive Risk Management Tools
Amplify your risk management with RegDesk’s integrated platform, designed to proactively identify and mitigate regulatory risks, including launch delays, compliance issues, operational inefficiencies, market entry barriers, and missed regulatory changes.
Global Regulatory Intelligence Access
Access essential global regulatory intelligence with RegDesk. Our platform not only keeps you informed of the latest regulatory changes and standards but also provides deep insights into emerging trends and compliance requirements, ensuring your operations remain up-to-date and fully compliant at all times.
Seamless Regulatory Software Integration
Effortlessly Integrate RegDesk into Your Existing Tech Stack With Our API
Allow our single interface to seamlessly connect with your existing systems such as SAP, eDMS, PLM, QMS, With streamlined workflows and better data synchronization, take your compliance operations to the next level.
See IntegrationsRecognized Excellence and Certifications
Trust a recognized leader. RegDesk is honored to be named a Gartner Representative Vendor for both Regulatory Information Management (RIM) and Regulatory Intelligence, reflecting our commitment to innovation in regulatory compliance solutions.
RegDesk is also recognized by Gens & Associates as a Regulatory Information Management (RIM) platform for medical device companies. RegDesk has also been listed as a Regulatory Intelligence provider.

SOC 2 Compliant

21CFR Part 11 Compliant

GDPR Compliant

GxP Compliant
Compliance Support Across Major Frameworks
With automated gap analysis, real-time intelligence, and submission tracking, reduce approval timelines and mitigate compliance risks. No matter the regulations, RegDesk’s integrated platform allows you to auto-generate forms required across major regulatory frameworks.
Ready to transform your regulatory processes?
Try our interactive demo and see firsthand how RegDesk can streamline your compliance operations. Eliminate your regulatory challenges now!
Request a DemoFrequently Asked Questions
What is regulatory compliance software?
Regulatory compliance software helps organizations comply with legal, security and other regulatory requirements. The software automates and streamlines processes to make sure internal policies are followed and external requirements are met. Common features of this type of software include tools for risk assessment, compliance tracking, audit management and reporting capabilities. In industries like healthcare, finance or manufacturing, where there are strict regulations, regulatory compliance software is a must. In order to reduce non-compliance penalties, ensure accurate reporting, and increase operational efficiency companies can deploy a centralized system so they stay updated with evolving regulations and requirements.
What is regulatory compliance for medical devices?
Regulatory compliance for medical devices refers to upholding laws and regulations designed by the governing bodies in relation to design, testing, production and marketing of medical devices. Governing bodies, for example, Health Canada or US FDA, want to ensure medical device safety and efficacy for patients so they set standards medical device companies must follow. Companies must adhere to strict compliance requirements like pre-market approval, clinical testing, post-market surveillance, and adverse event reporting, to legally sell their products in various countries. This process protects both manufacturers and patients while ensuring products are safe and reliable.
Why is a regulatory strategy in medical devices important?
A regulatory strategy for medical devices is important because it provides medical device manufacturers with a plan to navigate the complex regulatory landscape. With diverse requirements across different markets, it is imperative companies follow the most efficient path to market approval and compliance. Having a strategic plan in place allows companies to manage compliance risks, reduce costs and become more efficient in their regulatory submission and approval process. By ensuring regulatory obligations are met, companies can avoid costly delays and non-compliance issues which can provide a competitive advantage and build trust with stakeholders and patients alike.
What is the regulatory path for medical devices?
The regulatory pathway for medical devices varies across regions. Generally, the first step in this complex process is to determine the risk classification of the medical device because this affects the regulatory requirements and submission pathway. When you know the device classification and target markets, it is important to understand the regulatory requirements you must follow. There will be preclinical testing, risk assessment, clinical evaluation and evidence gathering that you will need to submit your application. Once you have all of your documentation, you must apply and obtain market approval. After approval, it is imperative that you follow post-market surveillance guidelines and report any adverse events which is an ongoing process for compliance.