The Therapeutic Goods Administration (TGA), the medical device regulating authority, has published a final consultation paper dedicated to the Australian Unique Device Identification (UDI) System to be implemented in the country in the course of the ongoing improvement of the Australian medical device regulatory framework.

Medical Device Regulation in Australia: Background
First, it is important to mention that nowadays the Australian Government develops and implements the program of the therapeutic goods regulation reform focused on the safety, performance, and quality of medical devices allowed to be marketed in Australia to ensure the highest level of public health protection. The TGA, as part of the Australian Department of Health, is taking steps towards the actual implementation of the regulatory reform.
The present TGA consultation paper on UDI regulations constitutes a final version of the document initially published by the authority to initiate a public discussion with the industry representatives and other parties involved in operations with medical devices, including the patients and healthcare professionals.
As it was already mentioned before, the initial consultation paper on UDI System to be implemented in Australia was published by the TGA earlier in January 2019. In particular, the authority suggested establishing the UDI System based on the requirements set forth by the UDI Application Guide developed by the International Medical Device Regulators Forum (IMDRF), a voluntary association of medical device regulating authorities. The scope of the consultation on the UDI System also covered the aspects related to the AusUDID – an Australian UDI database. The authority states that almost half a hundred submissions containing comments and suggestions. As it was stated in the submissions received, the industry representatives agreed with the importance of the UDI System implementation, and with the role the TGA should play in this process. They also confirmed that the AusUDID should be managed by the TGA and, moreover, linked to the Australian Register of Therapeutic Goods (ARTG) – the main Australian database for medical devices, medicines, and other medicinal products subject to registration. The respondents also agreed on using the aforementioned IMDRF guidance, together with the methods, approaches, and best practices adopted in other countries, as a basis for the new UDI regulatory framework. Besides the points above, the respondents also emphasized the following:
Australia should accredit internationally recognized Issuing Agencies (organizations that issue unique device identifiers for individual products), rather than establishing a new Issuing Agency.
- There is the need for clarity on who is responsible for submitting the UDI data into the AusUDID, especially where more than one sponsor holds pre-market authorization for the same device.
- Certain low-risk medical devices (e.g. Class I non-sterile medical devices without measuring functions) could be exempted from the UDI requirements. Moreover, some of the respondents also suggested expanding the scope of exclusion to other devices, such as custom-made, investigational medical devices, or ones distributed directly to end-users through retail stores.
It is also important to mention that the actual implementation of the UDI requirements in the EU would take place in several steps depending on the type of the device, namely:
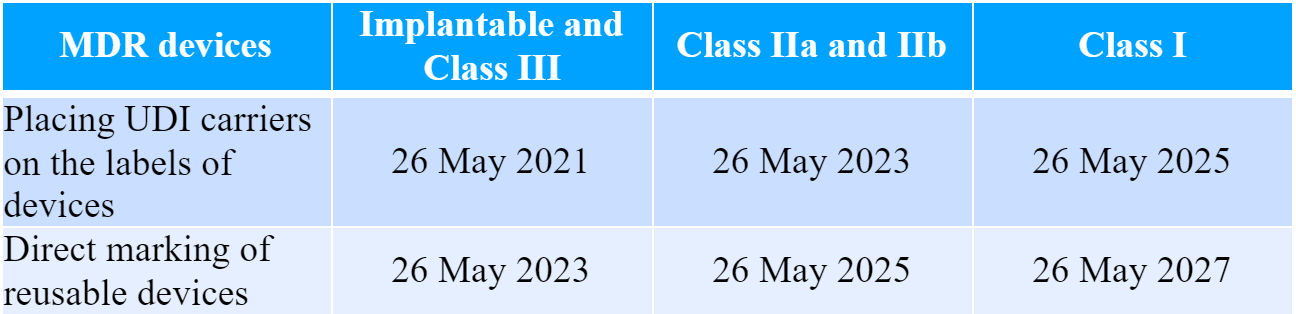
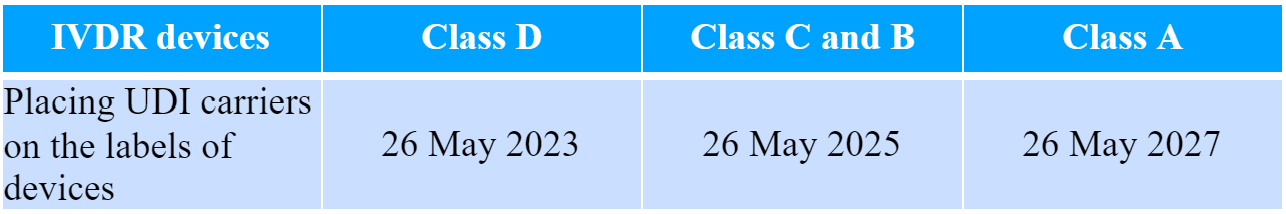
In the case of in vitro diagnostic medical devices the implementation schedule would be the following:
The TGA states that the development and actual implementation of the UDI system in Australia will take a significant period of time due to the numerous aspects to be considered. Thus, the authority intends to perform additional consultations related to the planning and design of the new System.
Basics of the Australian UDI System
The main purpose of the UDI System implementation is to improve the transparency and traceability of medical devices. The elements of the UDI System could be used in both pre-market and post-market stages. The proposed UDI framework covers all important aspects including the production of the UDI, its application on the label of the medical device or directly on the device itself, and also the maintenance of the UDI Database to be established to store the data related to the unique device identifiers.
It is planned that upon completion of the UDI System implementation, all medical devices allowed for marketing in Australia would be labeled with the UDIs served both in human and machine-readable form. The information on such devices would be also entered in the UDI Database (AusUDID). At the same time, the TGA also states that irrespectively of the requirements to be introduced in the context of the UDI system implementation, already existing requirements on the safety and performance of medical devices, including the Essential Principles set forth by the Medical Device Regulations would have the higher priority.
The TGA also outlines the benefits associated with the implementation of the UDI system. According to the consultation paper, the new unique device identification system would allow to:
- Improve the effectiveness of the actions associated with the recalls of medical devices, as well as the effectiveness of post-market surveillance in general due to the advanced identification opportunities it offers,
- Simplify access to the information related to medical devices marketed in Australia for the customers, and also to make all operations with such information more efficient and to reduce the number of errors and mistakes related to the information and data,
- Mitigate the risks associated with the potential shortages in medical device supply,
- Improve medical device identification in general.
UDI Issuing Agency
Another important concept associated with the UDI System is the Issuing Agency – an organization accredited by a regulatory authority to operate a system for the provision of UDIs according to specified global standards. At the moment, the list of the accredited Issuing Agencies includes:
- Global Standards One (GS1),
- The Health Industry Business Communications Council (HIBCC), and
- The International Council for Commonality in Blood Banking Automation (ICCBBA).
The aforementioned agencies have been accredited in numerous jurisdictions including the US and EU.
Each of the aforementioned agencies is entitled to assign the appropriate type of the UDI-DI (Device Identifier, a unique numeric or alphanumeric code specific to a model of medical device and intended to be used as an access key to the information related to the device and stored in the appropriate database), namely:
- Global Standards One – Global Trade Item Number (GS1 GTIN),
- Health Industry Bar Code – Labeller Identifier Code (HIBC LIC),
- International Society of Blood Transfusion – 12 Processor Product Identification Code (ISBT PPIC, ISBT-128).
Summarizing the information provided here above, the present TGA consultation paper on UDI System covers the most important aspects associated with the new unique device identification system to be implemented in Australia. The document describes the current viewing of the regulating authority regarding the UDI framework based on the best practices adopted in other countries.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:
https://consultations.health.gov.au/tga/unique-device-identification-udi-consultation-pape/

