May 24, 2016
North America
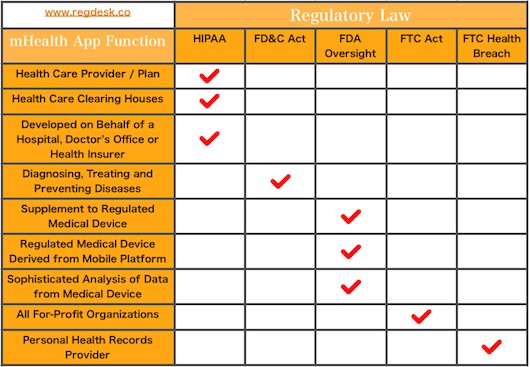
Mobile health app The growing significance of mobile health apps result in a need to ensure its proper regulation. Practitioners, patients, researchers and medical industries are all affected by mHealth apps. The digital health field is regulated by the FDA under four...
Read More

May 11, 2016
Consultant Post
China has become the second largest medical device market in the world. Sustained economy, population growth and a burgeoning aging population makes China a lucrative market for foreign medical device and pharmaceutical manufacturers. Nevertheless, China is considered...
Read More

Feb 18, 2016
RegDesk News/Info
5 tips for sending your medical device to sanctioned country: Contact OFAC First The most important piece of advice we can give to anyone who is interested in sending their device to a sanctioned country is to schedule a call with Office of Foreign Assets Control...
Read More

Jan 19, 2016
Asia
The Chinese Food and Drug Administration (CFDA) has issued a new “Medical Equipment Generic Naming Guide,” which will be implemented on April 1, 2016. This is the first regulatory document for ‘naming’ generic medical devices. The CFDA believes...
Read More

Jan 13, 2016
Asia
China has a Revised Drug GSP, ensure your
Read More
 North America
North America




