The Saudi Food and Drug Authority (SFDA), the country’s agency responsible for regulation on the sphere of medical devices and other healthcare products, has published a set of guidance documents dedicated to the relationships arising with regard to the importation and exportation of various radioactive materials intended to be used for medical purposes.

In particular, the new guidelines describe the requirements of importation and re-exportation for radioactive materials used in medical applications, as well as medical imaging materials. The documents are intended to provide medical device manufacturers, their authorized representatives, importers, distributors, and other parties involved in operations with medical devices with the additional recommendations and clarifications to be considered in order to achieve and sustain compliance with the applicable requirements set forth under the current legislation.

SFDA Guidance on Radioactive Materials
The first document issued by the SFDA addresses the matters retailed to the importation and re-exportation of radioactive materials intended to be used for medical and scientific research purposes. By the virtue of the document, the regulating authority highlights the most important requirements related to the safety and protection of patients.
Under the general rule, the SFDA would conduct the review of the appropriate request related to the use of radioactive materials by healthcare institutions. The regulating authority would also assess whether the materials in question are compliant with the applicable technical requirements.
In order to be allowed to use radioactive materials, an importer, exporter, or transporter shall:
- Create an account in the Unified Electronic System (GHAD) managed by the SFDA,
- Obtain a Medical Device Establishment License covering the activities in the sphere of radioactive materials, including their importation and re-exportation (to be granted by the SFDA),
- Submit the complete set of documents required by the regulating authority.
In case if the radioactive materials subject to importation and intended to be used in Saudi Arabia are classified as medical devices, the appropriate permission – Medical Device Marketing Authorisation – is required.
A party interested in importing or exporting radioactive materials shall submit the appropriate request in electronic form via the special system for radioactive materials – MRMR. Additionally, a hard copy of the application should be submitted to the Ministry of Interior, which would later forward the application to the SFDA for review.
For the purpose of the clearance in the port of entry, the interested party shall provide a copy of the manufacturer`s invoice, and also ensure the products are properly packaged, marked, and thus are identifiable. Additional information about general rules on customs clearance in the ports of entry could be found in the appropriate guidance issued earlier by the SFDA. The interested party shall also fulfill the requirements described in the guidance on storage, transportation, and dealing with medical devices & products published by the regulating authority.
The SFDA guidance on radioactive materials additionally describes the set of documents to be submitted by the interested party intended to import or re-export radioactive materials intended to be used for medical or scientific research purposes. According to the guidance, the documents to be submitted include, inter alia, the following:
- A copy of the Medical Device Establishment License allowing the interested party to carry out the activity in the sphere of importation and re-exportation of medical radioactive materials. Such a license is required for importers and exporters, while healthcare institutions could waive this requirement in accordance with the appropriate exemption.
- A copy of the Medical Device Marketing Authorization for radioactive materials subject to regulation as medical devices (if applicable).
- A copy of the Radiation Practice License issued by the respective regulating authority in the sphere of radiological control and supervision – either King Abdullah City for Atomic and Renewable Energy (KACARE) or Nuclear and Radiological Regulatory Commission (NRRC).
- A copy of the personal license of the Radiation Protection Officer License, together with a copy of the national ID or residence permit and agreement with the interested establishment.
- Copies of all the licenses of all the radiation safety officers employed by the interested entity, together with the documents specified above.
- A copy of the establishment license covering the transportation of radioactive materials.
- Letter from Public Security to the SFDA.
- Application form on importation or re-exportation of radioactive material filled electronically and printed on the letterhead of the interested entity. The appropriate template is also provided as an annex to the present SFDA guidance.
- A copy of the purchase order (in case of importing).
- A commitment letter from the manufacturer regarding the receipt of the radioactive material after being used (in case of exportation). This document shall indicate the name of the importer, and of the radioactive materials in question.
- A copy of the radioactive material transportation agreement, printed on the letterhead of the interested entity.
- A list of radioactive materials intended to be used for medical and/or scientific purposes, including the serial numbers of radioactive products. The document should be filled electronically and printed on the letterhead of the interested entity.
- A copy of the manufacturer Quality Management System (QMS) certificate in addition to the Good Manufacturer Practice (GMP) certificate (in case of importing).
- An official letter or free sale certificate confirming that the radioactive materials in question are allowed to be marketed in the country of origin (in case of importing).
- An acknowledgment stating that the radioactive materials are compliant with the applicable SFDA requirements on medical devices and their identification.
- Filled disclosure form.
- Filled undertaking form.
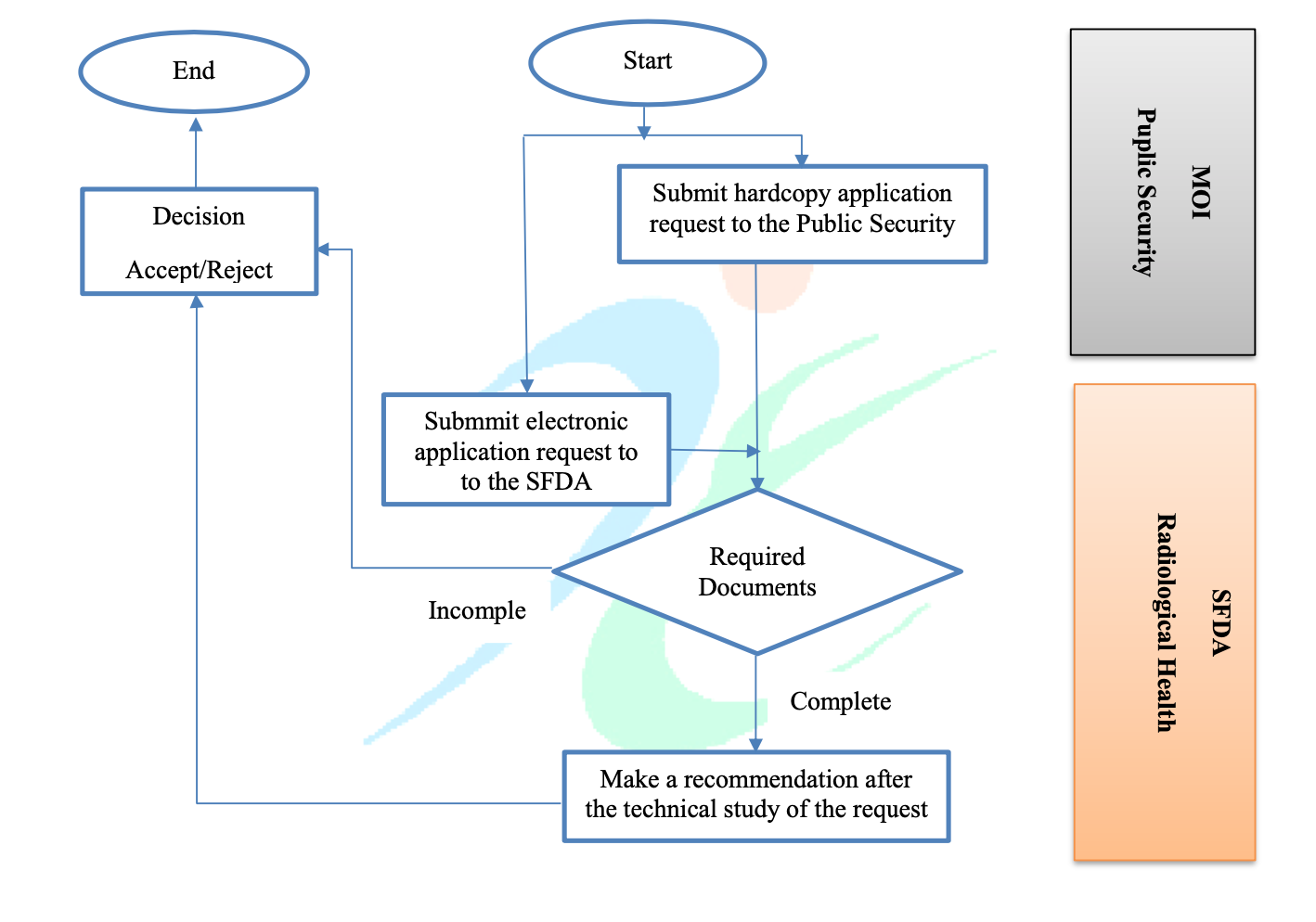
Application Flow
In order to assist medical device manufacturers, importers, distributors, and other parties involved in achieving and sustaining compliance with the applicable requirements described herein, the SFDA also provided a flowchart that describes all steps of the application process.
According to the guidance, the whole application process includes the following steps:
- Submission of the applications. As it was already mentioned before, a hardcopy should be submitted to Public Security, while the same application in the electronic form should be submitted to the SFDA.
- The applicant shall also submit all the documents required. Should the submission be found incomplete, the SFDA may reject the application.
- Upon submission of a complete application, the SFDA would conduct a technical assessment of the radioactive materials in question.
- If the applicant would demonstrate compliance with any and all applicable requirements, an acceptance decision would be made by the appropriate regulating authority.
When submitting the application, an interested party shall certify that information provided is complete and accurate. The radioactive materials subject to review should not be used for the purpose other than specified in the initial request. Moreover, all these products should comply with the applicable safety requirements and specifications. The content of the shipment should be accurately described by the interested party. The applicant shall also indicate whether the radioactive products in question are subject to regulation as a medical device (in such a case, further details would be required, including the name of a medical device and a registration number of a Medical Device Marketing Authorisation).
Summarizing the information provided here above, the present SFDA guidance on radioactive materials highlights the most important aspects related to importation and re-exportation of such products intended to be used for medical and healthcare purposes. The guidance outlines the scope of documents to be submitted by the interested party, and also the procedure to be followed in order to be allowed to import radioactive materials in Saudi Arabia.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:

