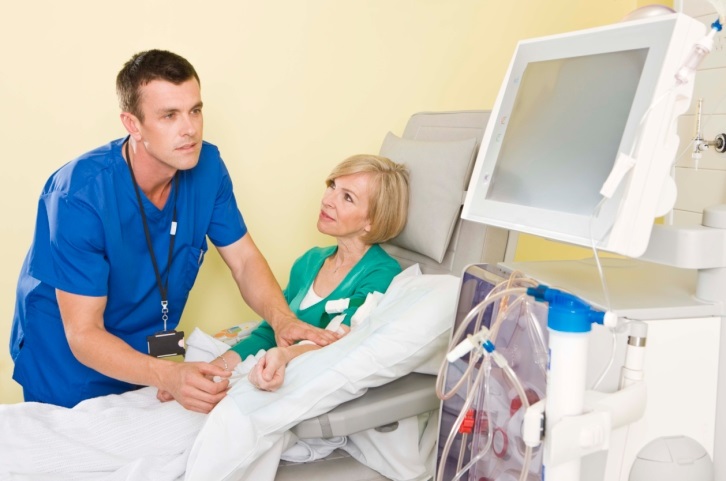
The new article describes in detail the scope of information about the device the authority expects medical device manufacturers to provide when applying for the authority’s approval. In particular, the article addresses the aspects related to the physical and electronic characteristics of systems, as well as their functions and features.

Table of Contents
The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of medical devices, has published a guidance document dedicated to the Investigational Device Exemptions (IDEs) for devices indicated for Nocturnal Home Hemodialysis (NHHD). The document describes in detail the regulatory approach to be applied for the said devices and also provides recommendations to be followed by medical device manufacturers to ensure compliance with the respective legislation. However, the guidance itself is non-binding in its legal nature, and it is not intended to introduce new rules or impose new obligations. Moreover, the authority explicitly states that an alternative approach could be applied, provided such an approach is in line with the applicable regulations and has been agreed with the authority in advance.
Physical and Electronic Description
As it was mentioned before, the device description to be provided by the medical device manufacturer should address the aspects related to the physical and electronic characteristics of the device in question. Since the devices of this type are intended for home use by laypersons, it is necessary to provide additional information regarding the way the potential user should interact with the device when using it for its intended purpose.
Apart from this, the manufacturer should provide details about the dialysate used in the system (including the indication of a particular type of concentrate used). In certain cases, it is also necessary to provide information about:
- The ration of the mixture of concentrates and water used;
- The minimum quality of water that is to be mixed with the dialysate;
- The type of concentrates that may be used (e.g., acid and bicarbonate, acetate); and
- The way incoming water is fed into the NHHD system.
The points listed hereinabove should be addressed in case the dialysis solution is to be prepared online, otherwise, the manufacturer is expected to provide a separate description of the type of solution, and the way it is to be prepared, stored, and delivered.
Apart from this, the manufacturers are encouraged to provide additional information about the water treatment system used to purify tap water. In this respect, the manufacturer should provide a detailed description of all the components of the system and the way they should be used in a home environment.
The manufacturers are also expected to describe all intended clinical configurations that are mentioned in the device labeling (e.g., single-needle, double needle) and explain which features of the system are used for each configuration. The manufacturer shall also provide the details related to the accessories that should not be used with the NHHD system in question due to compatibility issues. In case the system includes a medical device for which there is separate device-specific guidance issued by the FDA, the reference to such guidance should be provided accordingly. For the accessories, it is also important to include information about the accessories that are already marketed in the US and could be used with the system subject to review. For instance, if the system itself does not contain a tubing set, the manufacturer shall provide details about the tubing set which is already available on the market and is fully compatible with the system. The said information should be reflected in labeling, and also in the investigational plan for the respective trial. It is also stated that functional testing is to be conducted to validate compatibility with the tubing set manufactured by a third-party manufacturer.
System Features and Functions
Apart from the details regarding the physical and electronic characteristics, the authority also expects the manufacturer to provide information about the system features and functions. In particular, the manufacturer should submit a list of the performance specifications for the controllable parameters of the NHHD system, including both the range of allowable values and the default. The manufacturers are expected to provide details regarding such parameters as:
- Flow rate that each pump can deliver;
- Transmembrane pressure;
- Ultrafiltration rate;
- Dialysate temperature;
- Dialysate conductivity;
- Arterial and venous pressure;
- Length of treatment;
- The volume of water that can be purified, if applicable;
- Rate of production of purified water, if applicable;
- Water conductivity and purity, if applicable; and
- The volume of dialysate that can be processed.
Apart from the above mentioned aspects, the manufacturer is also expected to provide detailed information about the alarms the system has. The authority additionally emphasizes that since the NHHD system is usually used when the patient is sleeping, the alarm should be designed in a way ensuring it can awaken the patient in case it is reasonably necessary to prevent issues. The manufacturer shall provide a detailed description of each of the alarms, and also identify the following matters:
- The hazard condition that triggers the alarm;
- How the subject or the trained care partner should respond to and resolve the condition that triggered the alarm; and
- How the machine responds to the alarm condition (e.g., system shutdown).
The FDA also mentions that the potential users are the ones who are expected to interact with the device in general and alarms in particular, the description provided should reflect the human factors or usability. The guidance also refers to the respective standard to be considered concerning the alarm system.
In case the device has a remote monitoring system, a detailed description of such a system and the way it operates should be provided by the manufacturer as well.
The authority also provides a list of key design features the NHHD system should have to be used safely and efficiently. This includes, inter alia, the aspects related to the use of the system for its intended purpose, as well as maintenance and incident prevention.
In summary, the present FDA guidance outlines the scope of information the medical device manufacturer shall provide for the physical and electronic characteristics of the NHHD system, as well as its functions and features. The document highlights the key points to be considered to ensure the authority is provided with all the information reasonably necessary to assess the safety and effectiveness of the system, and also to ensure that all the important information is duly communicated to potential users.
Sources:
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.