The Medicines and Healthcare products Regulatory Agency (MHRA) of the UK has published guidance dedicated to the medical device stand-alone software including applications. The scope of the guidance covers standalone software and applications intended to be marketed in the UK. The MHRA additionally mentions that the medical devices marked with the CE mark would be allowed for marketing in the UK until June 30, 2023. The guidance is provided in the form of an interactive document to be viewed in electronic form and includes internal links. The document describes current regulatory requirements and also provides additional recommendations to be considered by the medical device manufacturers and other parties involved.

Regulatory Background
The MHRA acknowledges the increasing importance of medical device apps. Nowadays they are widely used in the healthcare sphere for various purposes. According to existing regulatory requirements, both medical software and apps should have UKCA conformity marking as prescribed by the Medical Device Regulations 2002. The aforementioned marking confirms the medical software is compliant with the applicable regulatory requirements in terms of safety and effectiveness.
One of the most important questions arising with regard to the medical software and apps relates to the determination of whether the particular application subject to review shall be treated as a medical device or as one intended for general health and fitness purposes. The present MHRA guidance is intended to clarify this issue. For this purpose, it contains various examples, and also some flowcharts describing the approach to be applied when making such a determination. The document also describes the regulatory requirements related to classification and marking. The recommendations provided in the guidance should be considered by the medical software developers before placing their product on the UK market.

Use of a Medical Software – General Points
The MHRA guidance provides recommendations for persons using medical software. According to the document, before using the software, they should ensure it has the necessary marking – CE or UKCA. The two main types of medical devices are:
- The ones calculating medicine doses for the particular use, or
- The ones indicating the medical condition a user may have, or the individual percentage of such risks calculated on the basis of the information provided by the user.
The MHRA also states that it is important for the user to understand the information the app provides, and also the actions to be taken further in this regard. These criteria should be applied when choosing a particular medical app to use depending on its intended purpose. When using the app, the user shall follow carefully the instructions for use provided by the developer of the app. The regulating authority additionally emphasizes on the importance of the accuracy of the information the user inputs into the software since it would impact the accuracy and reliability of the result the app would give. The user shall also ensure he/she is using the latest version of the app.
In case if the user has certain concerns regarding the results provided by the app or the way they should be interpreted, he/she shall contact a healthcare professional to consult with. In certain cases, the user shall also contact MHRA, namely:
- The instructions provided by medical software developed are unclear,
- The app does not provide the results it has provided,
- There are certain concerns with regard to the safety of the app or the information provided.
Should any of the cases indicated hereabove take place, the MHRA recommends the user to contact the regulating authority, and also to inform the medical software developer.
Another important aspect related to medical software and apps is the protection of personal data the user puts into the app. The MHRA states that the user shall clearly understand the scope of personal data he/she agrees to share with the medical software developer when using the app, and also the way the data would be stored and processed, especially in terms of sharing with any third parties. The information the user usually provides to the app is quite sensitive since it includes not only the name, address, date of birth but also the details about health.
Device Determination Flow Chart
As it was already mentioned before, the present MHRA guidance on medical software and apps provides recommendations regarding the way the regulatory status of a software product should be determined. For this purpose, the document provides a flow chart to be used in case of software not incorporated in a device. The first eligibility criteria to be met states that it should be a computer program or functional document.
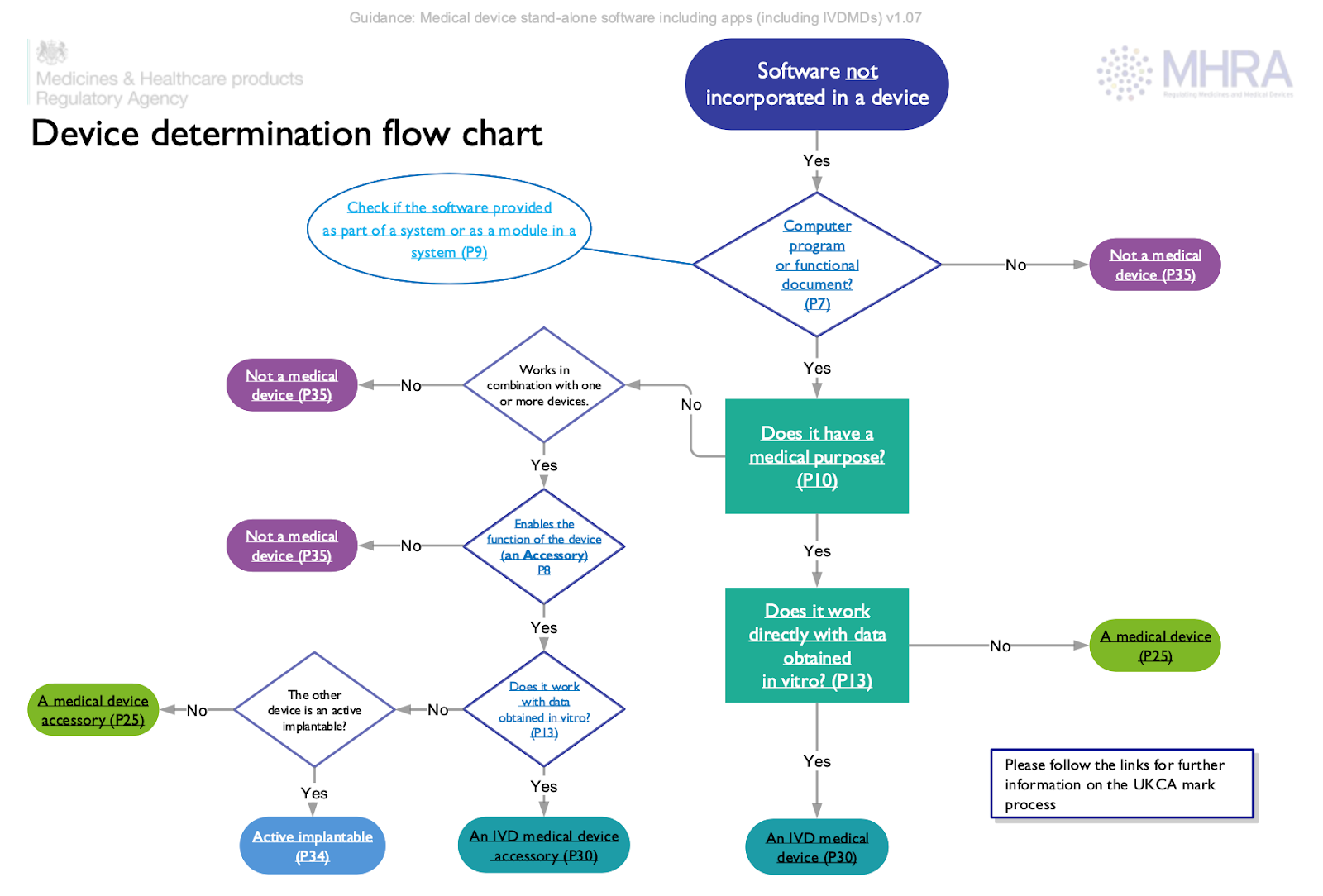
According to the flow chart, the product subject to regulation under the applicable framework include:
- Medical devices,
- Accessories for medical devices,
- Active implantable medical devices,
- In vitro diagnostic (IVD) medical devices,
- Accessories to the IVD medical devices.
In order to assist medical software developers and other parties involved in applying the principles stated hereabove, the MHRA also provides the definitions of the main concepts to be applied in this regard, namely:
- Computer program – a syntactic unit that conforms to the rules of a particular programming language and that is composed of declarations and statements or instructions needed to solve a certain function, task, or problem.
- Functional document – a software that requires separate software to perform its functions. Often this will be a general-purpose application. For instance, this could be a document containing a flow chart or script, a spreadsheet, or interactive web pages.
- Accessory – a product intended to enable a medical device to fulfill its intended function and it will be treated as a device under the UK MDR 2002. At the same time, in case if the app in question constitutes the only way to interact with a physical device, such an app could be considered as a component of the device. In such cases, the app could display, store and analyze the data collected by the medical device.
The MHRA also mentions that whilst there is no special definition for a system in the context of medical devices regulation, there are specific regulatory requirements to be applied. As it is stated by the regulating authorities, a set of products placed on the market together and intended to be used together should be considered as a system, even if some of these products are not medical devices.
In certain cases, the UKCA marking could be required for the whole system, but only for some of its modules and functions – the ones that meet the definition of a medical device under the current regulatory requirements.
Intended Purpose of a Medical Software or App
The MHRA also emphasizes the importance of the intended purpose of a product in question as one of the criteria to be applied when determining its regulatory status. In order to assess this, the regulating authority would refer to the claims made by the medical device manufacturer, as well as the labeling of the device, instructions for use, and promotional materials used. Thus, the medical software manufacturer should pay attention to the accuracy of the claims made – the intended purpose of a product should be described as precisely, as possible, to avoid incorrect determination.
Summarizing the information provided here above, the MHRA guidance on medical software and apps provides additional clarifications on the applicable regulatory requirements, as well as additional recommendations to be considered by the medical software developers and other parties involved. The guidance also contains a flow chart to be applied when determining the regulatory status of a software product before placing it on the market.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:

