Regulatory Information Management System (RIMS)
Transform Regulatory Compliance with an AI-Powered RIMS Solution

If Any Of The Following Sound Familiar, You Need RegDesk
RegDesk’s RIMS delivers a holistic solution to meet all your regulatory team’s needs in one centralized platform
We evaluated many existing platforms. We chose RegDesk because it addresses all our regulatory needs from regulatory intelligence to helping us prepare applications and manage change assessments.
Regulatory Affairs Specialist, Fortune 500 Company
The Ultimate Guide to all things Regulatory Information Management Systems (RIMS)
What is a Regulatory Information Management System (RIMS)?
RIMS is a widely accepted solution for pharmaceutical products. In fact, most RIMS are built for pharmaceuticals. But the medical device and medtech industry is just as complex and needs the kind of lifecycle management that only a medical device specific RIMS can provide.
Why does the medical device product lifecycle need its own RIMS?
The medical device and medtech industry has seen great demand and innumerable regulatory changes in the last few years. The market value for medtech is estimated to reach $745 billion by 2030, and today, there are an estimated 2 million different kinds of medical devices globally, categorized into more than 7000 product groups. Medtech businesses are realizing that legacy systems, manual processes, and outdated solutions will not help them scale their operations and meet such demand.
The medical device industry is heavily regulated to ensure medical devices are safe for patients. The regulatory governance scene changes daily, with new and evolving regulations, especially around AI, cybersecurity, privacy, and other technology. In a recent survey, 87% of respondents identified government regulations as a threat and disruptor to their medtech business.
Regardless of the class risk of products — whether a toothbrush or a heart stent — all medical products must be approved by the local Ministry of Health before they can be marketed or sold. For instance, bringing a medical device to the European market requires compliance with regulations set by the European Union Medical Device Regulation, while in the United States, teams must comply with rules from the Food and Drug Administration.
Regulatory affairs teams prepare applications or dossiers for submission to health authorities to obtain approval. These teams must compile massive amounts of documentation and technical files specific to each market, which is laborious. On average, it takes regulatory teams between four to six months to prepare, format, and publish a single product submission for a country. The ever-increasing complexity of global medical device registration regulations is why many new, potentially life-changing medical technologies end up in regulatory limbo.
Gaining the health authorities’ approval also takes quite a bit of time: The average approval time is 9 months to a year. However, depending on how stringent the regulations are in some countries, it can take well over two years to get a single product approved. The higher the risk class of the medical device, the longer the approval time. This also means that regulatory bodies might need more data to be convinced that the product is safe and effective.
However, despite how rapidly the medical technology world changes, regulatory affairs in medical device companies are still far from digitized: Regulatory professionals in the medical device industry still use spreadsheets, printed-out documents, and other disjointed and outdated tools and processes to manage all their records and workflows.
|
Feature |
Traditional Methods |
|
Document Management |
Manual data entry, spreadsheets, inconsistent formats |
|
Workflow Management |
Manual routing of documents and tasks |
|
Task Automation |
Manual execution of all tasks |
|
Compliance Management |
Manual review for compliance, risk of errors |
|
Collaboration |
Limited collaboration due to reliance on physical documents or email |
|
Version Control |
Manual version control, risk of using outdated documents |
|
Submission Preparation |
Manual eCTD assembly, increased risk of errors |
|
Reporting and Analytics |
Limited visibility into the submission process, reliance on manual reports |
|
Scalability |
Limited scalability, additional resources needed for manual processes |
|
Regulatory Intelligence |
Limited access to real-time regulatory information |
There’s a case to be made for a collaborative platform aimed at collecting, organizing, managing, and analyzing regulatory information for the medical device industry.
Another large roadblock is that most companies have less than 15 regulatory professionals handling these complex processes. Especially with the labor shortage in regulatory affairs, it becomes crucial that Medtech companies find solutions that streamline and automate tasks instead of adding complexity. That’s where a RIMS comes in.
What is the importance of holistic RIMS for medtech?
The lack of a comprehensive analytics and information management system hinders innovation. It leads to a lack of communication and administrative complexity, costing the healthcare industry over $260 billion in unnecessary spending.
Regulatory compliance is an organization-wide process. From ideation to engineering to launch, there are records, paperwork, documentation, and processes that every team in an organization must maintain. The more relevant documents you submit to regulatory bodies, the sooner you get approval — and the sooner you release a product in the market. The regulatory submission process is complex, but it can be streamlined. The same information may be required by different markets. While no single harmonized global application exists for medical devices, identifying the similarities between applications is the key to streamlining submissions.
A Holistic RIMS
- increases regulatory intelligence in real-time
- improves the quality of interactions with regulatory governance authorities
- streamlines the flow of data between different functions in the organization
- automates complex processes and saves time
- provides valuable insights from the data
What is a Traditional RIMS vs. holistic RIMS?
In complex application processes, the only thing regulatory teams can control is how fast they can submit the applications to the authorities. However, outdated regulatory compliance risk software holds them back.
Many regulatory and compliance solution companies aim to help regulatory teams navigate the nuances of regulatory compliance in different markets. To that end, they provide templates and host applications to help teams stay organized but don’t solve the root of the issue: redundancy.
Traditional RIMS software requires teams to manually input information for every application, even if that data has been uploaded elsewhere. While it may be easier to house applications all in one place, manually entering data for each application still makes for a redundant, time-consuming process. The more manual input required, the more the opportunity for errors.
It’s crucial to remember that investing in multiple disconnected solutions that multiple teams use only widens the gaps in data. From resource allocation and building expertise in new technology to ensuring integration with the existing platforms and maintaining multiple solutions, it only contributes to additional data silos.
What are the advantages of a holistic RIMS?
While most RIMS platforms simply host applications, a holistic RIMS powered by AI guides users through submission. In such a platform, the application of AI for regulatory compliance touches every part of the submission process to help teams truly.
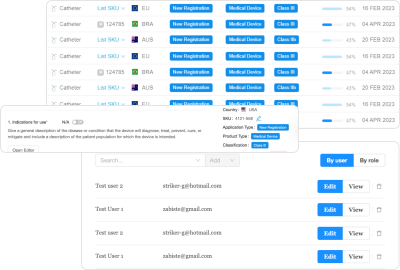
A holistic RIMS conveniently consolidates submission requirements into simple, fillable forms and leverages the power of AI to take things one step further and auto-populate subsequent submissions. Such a platform utilizes AI to automatically prepare jurisdiction-specific submission dossiers based on product and regional targeting information. Simply put, it archives data from previous submissions and uses it to autofill new applications for other markets.
Regulatory teams can drastically reduce the time it takes to complete forms for different markets while ensuring compliance and mitigating human error. They can utilize an AI-powered form-building tool to prepare arduous long application forms such as GSPR, Essential Principles, and Declaration of Conformity smarter and faster.
A holistic RIMS solution with AI collects and assembles the necessary data, documents, and evidence for medical device registration submission. This eliminates redundancy of effort for regulatory teams and makes it easy for international affairs regulatory teams to prepare and publish high-quality submissions within a few hours. It handles the behind-the-scenes coding and logic, so regulatory teams are free to focus on other important matters. What once took several months with many arduous tasks now only takes a few hours.
What are the advantages of AI-powered RIMS for medtech?
- Once the product is approved, they must start monitoring the products in the market to ensure no adverse events.
- They also have to monitor and track whether the approved product is undergoing any changes, such as changes to labeling, intended use, manufacturing, etc.
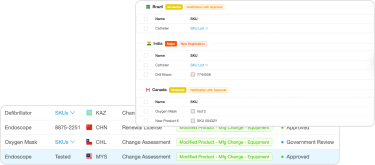
- They must ensure that the product approval license is renewed before expiration. If the license expires, you can no longer sell the product.
- The team must monitor any changes in regulations or standards after the product has been released. They must also perform an impact assessment to show the health authorities how the changes affect the product and what the regulatory impact is, which can initiate a whole host of new tasks.
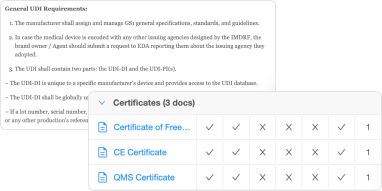
Organizing regulatory requirements
- Reducing the risk of non-compliance with up-to-date regulatory requirements integrated into the platform
- Centralized repository of regulatory documents, guidelines, and standards
- Management of regulatory changes and updates
- Following up on changes with necessary steps
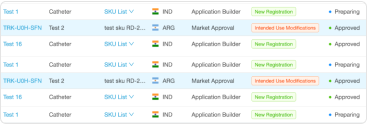
Managing submission procedures
- Preparing the relevant premarket submissions for the product’s classification
- Optimization of the submissions process according to device types, with only the steps necessary
- Automatically checking the completeness, correctness, and consistency of data for submissions
- Following up on submission measures
- AI-powered assistance in the submission of regulatory applications and documents
- Management of technical files
- Monitoring compliance deadlines and tasks
Post-launch monitoring and ongoing regulatory intelligence in real-time
- Automated collection and filtering of data
- Automatic analysis and trend calculation of the data
- Support in the preparation of reports
- Assistance with any follow-up steps required
- Reporting and analyses in the area of regulatory information across all your target markets
- Continuous learning and improvement from information provided by regulatory teams to further optimize processes
- Enforcing strict version control mechanisms
Ultimately, a holistic AI-powered RIMS automates most of these steps, reducing inefficiencies and the potential for human error.
|
Feature |
Updated Methods with an AI-powered RIMS |
|
Document Management |
Centralized digital repository with advanced search and real-time accessibility |
|
Data Management |
Automated data integration, standardized templates, and real-time updates |
|
Workflow Management |
Automated workflows with customizable approvals and notifications and automated triggers |
|
Task Automation |
AI-driven task automation reducing time and manual effort |
|
Compliance Management |
Proactive compliance checks with integrated validation and regulatory updates |
|
Collaboration |
Cloud-based platforms enabling seamless global team collaboration |
|
Version Control |
Automated version control with audit trail ensuring the latest documents are always in use |
|
Submission Preparation |
Streamlined eCTD creation and submission with error-checking tools |
| Reporting and Analytics |
Real-time analytics dashboards and predictive insights |
|
Scalability |
Scalable to accommodate growing data volumes and regulatory requirements with minimal resource increase |
|
Regulatory Intelligence |
Integrated access to global regulatory databases and real-time updates |
What is the impact of AI-powered RIMS for medtech?
- Cut down their regulatory strategy timeline by over 80%
- Reduce product evaluation costs by over 60%
- Reduce regulatory risk by 75%
- Save almost 12 months by reducing the number of hours per submission by improving accuracy and quality
- Triple the global output in just two years while maintaining a lean team











