The Health Products Regulatory Authority (HPRA), the Irish regulating authority in the sphere of medicines and medical devices, has published a draft guidance document dedicated to new applications for wholesale distribution authorizations (WDAs), as well as variations thereto. The document also refers to separate guidance documents describing in detail the general regulatory requirements with regard to wholesale and good distribution practices to be applied in Ireland. The authority additionally emphasizes that the applicant should be ready for the appropriate inspection to be carried out when assessing the application, otherwise the latter would be found incomplete. Moreover, as it is stated by the HPRA, any variations to existing authorization should be approved by the regulating authority in advance.

Regulatory Background
Under the general rule, any and all entities engaged in the distribution of healthcare products received directly from third countries should apply for the appropriate authorization in order to be allowed to market such products in Ireland. At the same time, the wholesale distribution authorization does not cover such cases. According to the guidance, a WDA applies for further distribution of healthcare products by entities other than the ones manufacturing them.
The HPRA outlines the eligibility criteria an interested entity should meet. According to the document, the wholesaler must have a permanent physical site in Ireland where the wholesale activities take place, for which the WDA will be valid. Wholesale activities must take place at the site and the necessary equipment to conduct the wholesaling activities must be located there. The wholesaling site must be accessible at all times to the HPRA.
Moreover, the authority states that the appropriate records related to the activities carried out by the wholesaler should be available to be provided upon request. According to the document, these records should be available at the site. Should the record-keeping be performed in electronic form, all records should be also available at the site through the equipment of the wholesaler.
Another important eligibility criterion relates to the personnel the wholesaler should have. As it is stated in the document, there should be at least one staff member in the country at the site where the activity takes place, with access to the aforementioned records. This staff member should be available at all times, even in case of an unannounced inspection of the regulating authority. The contact details of this staff member should be duly communicated to the regulating authority.

Application for Wholesale Distribution Authorisation
In order to obtain a Wholesale Distribution Authorisation, an interested entity shall submit the appropriate application comprised of necessary documentation requested by the regulating authority. For instance, the applicant shall provide the documentation related to its registered address. According to the document, the registered address of the applicant entity could be different from the address of the site where the wholesale activities would actually take place. The applicant should also provide confirmation of the business name registration. Thus, the address where the activity would actually take place is the first aspect to be considered by the applicant requesting a wholesale distribution authorization.
Variations to Wholesale Distribution Authorization
As it was mentioned before, the present HPRA guidance also describes the aspects related to variations to existing authorization. In this regard, the document provides a detailed description of the actions to be taken in each particular case, as well as the timeframes for the appropriate variations.
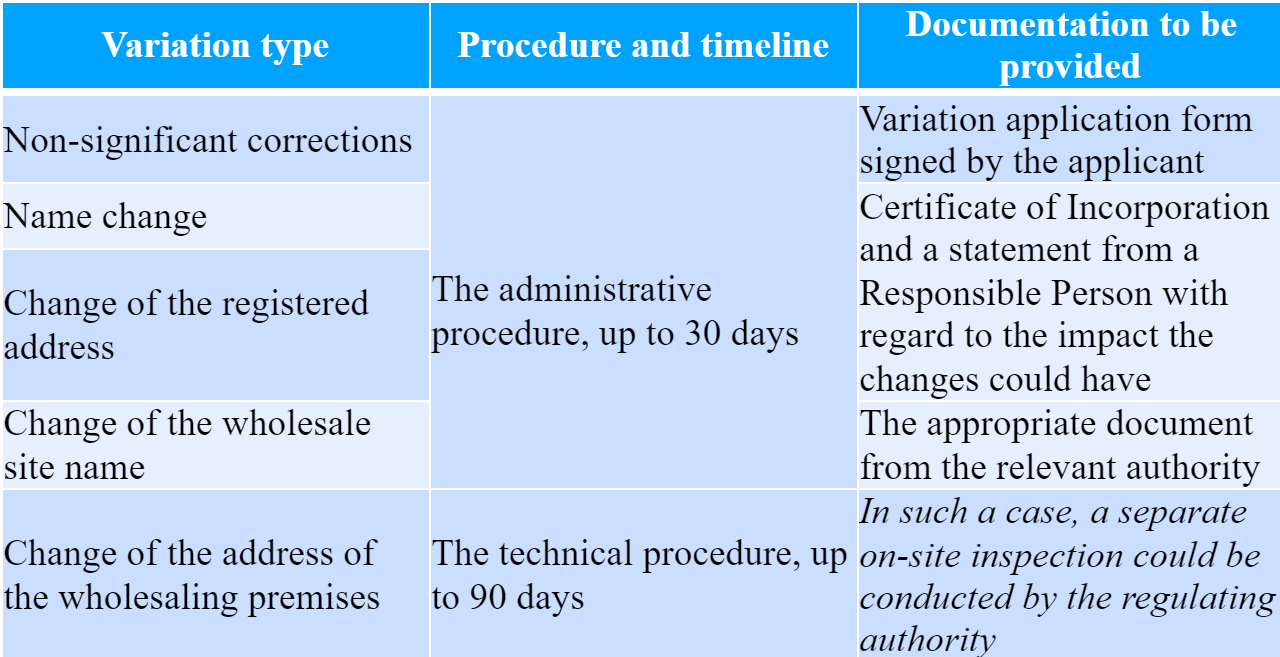
The document also describes in detail the way the variations should be performed with regard to each particular element of the wholesale distribution authorization, namely:
- Product types. The wholesale distribution authorization outlines the types (categories) of medicinal products the authorization holder is entitled to distribute. The addition of a new category would require a technical procedure and would take up to 90 days, within which a separate inspection of the regulating authority could take place. At the same time, the removal of one of the categories requires an administrative procedure to be performed within 30 days without any additional regulatory requirements to be applied.
- Permitted procedures. The WDA also contains information about the particular procedure the authorization holder is entitled to be performed. Such procedures could include, inter alia, the following ones: Procurement, Holding, Supply, Export. In the case of Procurement and Supply procedures, the applicant shall demonstrate compliance with the applicable regulatory requirements, especially in terms of the title of the products to be supplied. In this regard, the applicant entity shall provide a detailed business model describing the way the activity would be conducted. The regulating authority additionally emphasizes that such a model should describe the transfer of title – the applicant entity should be one of the medicinal products in question at the moment of distribution. As in the case of medicinal products categories, the addition of the new operation requires a technical procedure (90 days), while for the removal an administrative procedure should be applied (30 days).
- In case of distribution of certain medicinal products subject to special regulatory requirements, a separate license/authorization could be required (e.g. wholesale of controlled drugs). The document provides a list of such products to be considered by the interested party applying for a wholesale distribution authorization. Variations to these categories require a technical or administrative procedure respectively.
Wholesale Distribution Sites: Regulatory Requirements
The present document also provides additional clarifications with regard to regulatory requirements to be applied for wholesale distribution sites to be used by the interested party to conduct its activity. According to the guidance, an entity applying for the aforementioned authorization should provide the following details:
-
- The names and addresses of all sites, together with the appropriate authorization numbers;
- Confirmation of authorization issued by the relevant authority for each site located outside Ireland;
- A contract storage/distribution site must be included for all applications for Procure and Supply only activities;
- Confirmation of a technical agreement;
- Confirmation of successful completion of an audit in terms of Good Distribution Practices;
- Confirmation demonstrates that all special authorizations are in place for any and all products to be distributed.
Summarizing the information provided here above, the present HPRA guidance provides additional clarifications with regard to the regulatory requirements for the wholesale distribution of medicinal products. The aspects the regulating authority emphasizes should be considered by an interested party applying for such an authorization. The document also outlines the eligibility criteria an interested party should meet.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:

