The new article highlights the aspects related to the device description to be included in premarket notification submissions under the respective legal framework.

Table of content
The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has published a guidance document dedicated to premarket notification submissions for magnetic resonance diagnostic devices (MRDD).
The document provides an overview of the applicable regulatory requirements, as well as additional clarifications and recommendations to be taken into consideration by medical device manufacturers and other parties involved in order to ensure compliance thereto.
At the same time, provisions of the guidance are non-binding in their legal nature, nor are they intended to introduce new rules or impose new obligations.
Moreover, the authority explicitly states that an alternative approach could be applied, provided such an approach is in line with the existing legal framework and has been agreed with the authority in advance.
In particular, the guidance outlines the aspects to be covered by the device description to be submitted in the context of a 510(k) premarket notification for an MRDD.
The FDA has set clear guidelines for the 510(k) premarket notification process, as per 21 CFR 807.87.
This article provides a thorough exploration of these guidelines.
Indications for Use
First of all, the document covers the aspects related to the indications for use (IFU) in the context of MRDDs intended to be marketed and used in the US.
According to the guidance, the appropriate submission should cover the following aspects:
- Description and Consistency : It is mandatory to include the specific indications of your device, known as the IFU.
Every piece of material associated with the MRDD, be it for training, promotions, or performance claims, should strictly align with the stipulated IFU. - Clinical Indications : The document avoids addressing particular clinical indications, such as disease identification or prognosis.
If the device in question targets such specific indications, a proactive consultation with the Agency is strongly advised. Additional information could be derived from clinical studies. - Protocols : MRDDs might be equipped with distinct protocols tailored for specific applications. However, these protocols should not be misconstrued as new indications unless they make explicit disease-specific claims.
- Compatibility : For RF coils and accessory devices, it is vitally important to define which MRI systems they are compatible with, often detailing the manufacturer and field strength of the MRI system.
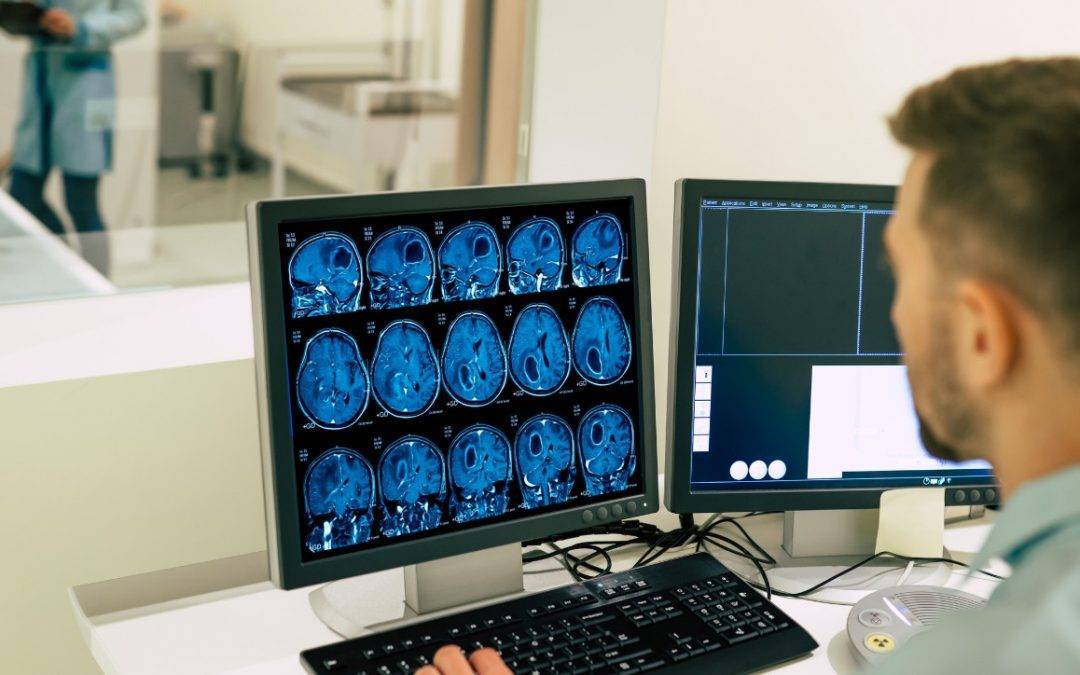
Device Description
The document further provides additional clarifications regarding the device description the authority expects to be provided. In particular, the following components require detailed elaboration:
- Magnet :The medical device manufacturer is expected to provide an exhaustive depiction of the main magnet, encapsulating its type, strength, dimensions, and its design features, as well as specifics such as weight, bore dimensions, and shielding.
- Gradient System : The gradient system should be elaborated upon, highlighting its physical characteristics and functionalities.
Crucial details would include shielding, maximum gradient amplitude, and control measures for cardiac and peripheral nerve stimulation. - Radiofrequency (RF) System: A thorough overview of the RF transmit-receive system’s architecture is needed. The FDA emphasizes the inclusion of the quadrature transmit mode in MRDDs.
- RF Coils : Each RF coil that is part of the system subject to review requires individual detailing, from its type and hardware specifics to its design and intended application.
- SAR Management and Control System: This section should provide a detailed description of how the Specific Absorption Rate (SAR) is managed and controlled. The console’s SAR values should be accurate, and the method of relaying energy deposition details to users should be transparent.
- Pulse Sequences : The term “pulse sequence” refers to techniques such as spin echo or echo planar imaging. Each sequence in the MRDD should be depicted in depth.
- Imaging Protocols: Each imaging protocol, essentially a workflow tool combining multiple pulse sequences, should be described in detail, elucidating its purpose and components.
- Image Processing : This section covers the algorithms working on acquired image data, detailing the functionality, user interaction level, and outputs of each image processing feature.
- Software : The application should be accompanied with software documentation tailored to the unique characteristics of the device.
The authority additionally emphasizes the importance of cybersecurity, especially if the device in question falls under section 524B(c) of the FD&C Act. - Accessories :An exhaustive list of accessories accompanying the system, such as physiological monitors, should be presented.
- Additional Information : This section encompasses details such as the patient table’s description, communication tools for patients, and RF shielding specifics.
Conclusion
In summary, submitting an MRDD for the 510(k) premarket notification is a meticulous process requiring adherence to the appropriate guidelines issued by the FDA.
Furthermore, medical device manufacturers are encouraged to get in touch with the authority to discuss additional details before making the submission.
How Can RegDesk Help?
RegDesk is an AI-powered Regulatory Information Management System (RIMS) designed to simplify global compliance for medical device companies. With regulatory intelligence covering 120+ markets, RegDesk helps you prepare and publish global submissions, manage standards, conduct impact assessments, and stay ahead of regulatory changes all from a single, centralized platform. Expanding into new markets has never been easier.