RegDesk Regulatory Intelligence Software
Stay Current on Regulation Changes Globally
With easy-to-understand regulatory requirements from over 120 markets and daily alerts, your team won’t scramble to remain compliant with new laws or regulations. RegDesk’s real-time, integrated regulatory intelligence platform gives your team control and clarity to address current and upcoming regulations globally.
RegDesk Alerts saved our regulatory affairs teams unprecedented amounts of time. Eliminating spreadsheets and automating regulatory intelligence has been a great business decision for us.
Liz H., Senior Director of Regulatory Affairs,
Medium-Size Medical Device Company
With RegDesk’s Medical Device Regulatory
Intelligence, You Can:
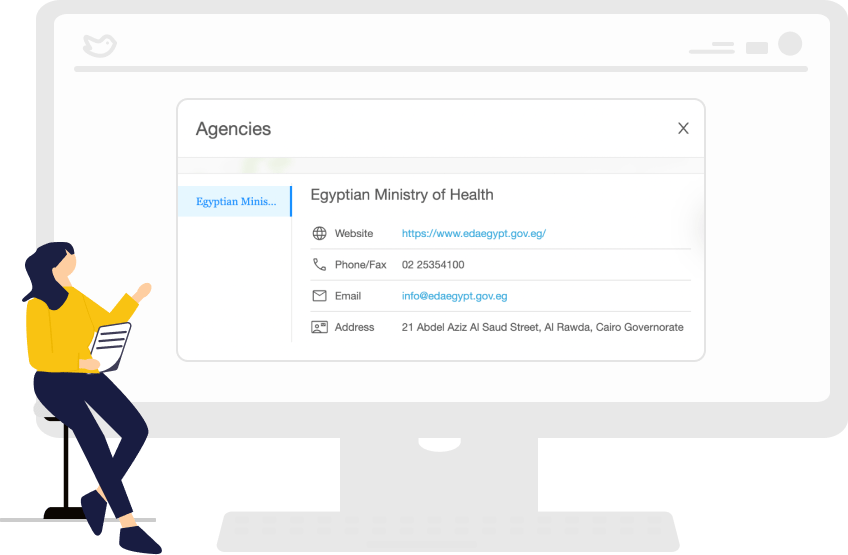
Access comprehensive regulatory requirements for 120 markets
Medtech and Pharma companies can now access the most up-to-date regulations in more than 120 markets. In addition, our regulatory intelligence tool eliminates hours wasted on search engines and endless email chains between regional teams. Instead, all the relevant information you need is accessible at the click of a button.
Receive Daily alerts on evolving changes
Daily alerts ensure that you stay informed of the latest developments and changes globally. The alerts enable you to respond quickly and proactively to any changes that may occur.
In addition, alerts provide you with a glimpse into all the forthcoming new regulations, providing you with ample time to prepare accordingly.
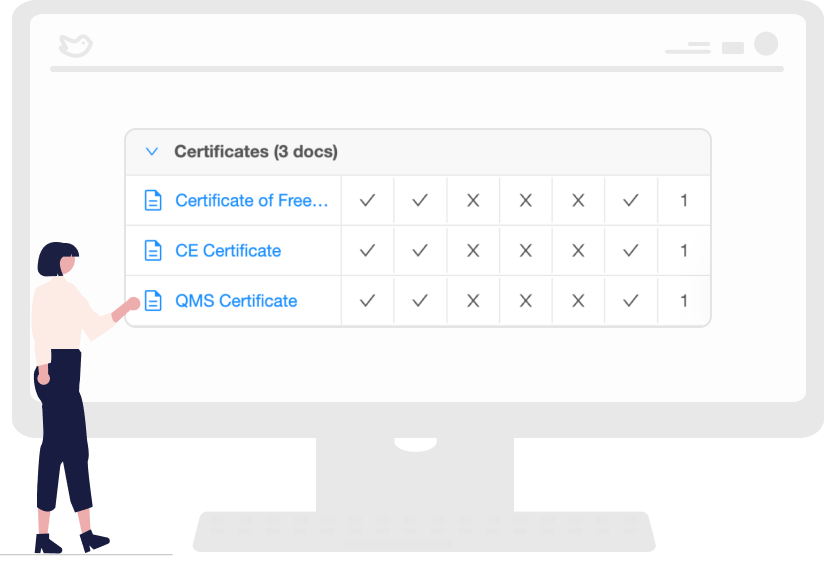
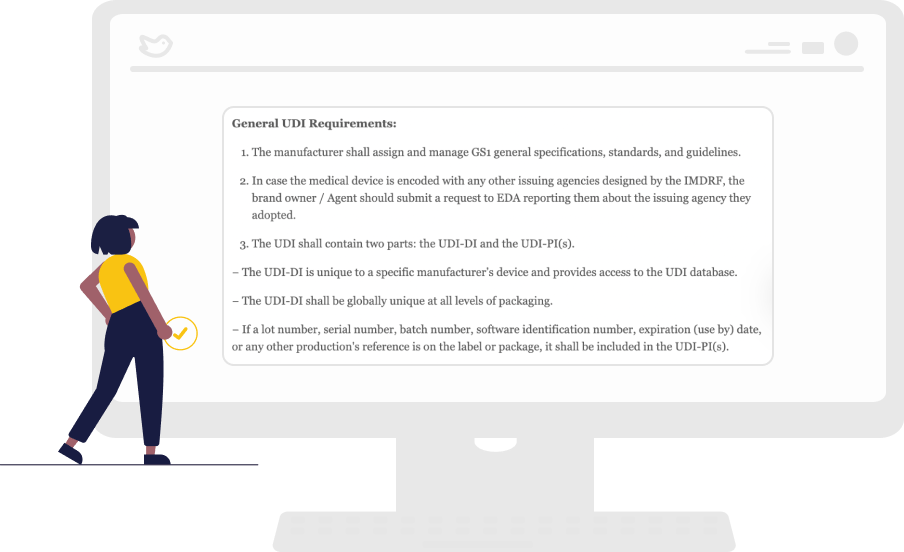
Regulatory Requirements that are Easy to Understand
The regional legislations are often in its local language, and online translation tools often do not work. Our platform provides regulatory teams with easy-to-follow requirements checklists with timelines and fees. In addition, it synthesizes the regulations in English.
Regulatory Requirements that are Easy to Understand
The regional legislations are often in its local language, and online translation tools often do not work. Our platform provides regulatory teams with easy-to-follow requirements checklists with timelines and fees. In addition, it synthesizes the regulations in English.

Respond to Regulatory Changes
Don’t let regulatory changes catch your team off guard. RegDesks’ regulatory intelligence software gives your team the insight they need into changing regulations in over a hundred markets. Find the relevant information you need to respond instead of react to change all while remaining compliant.
Who Needs a Medical Device Regulatory Intelligence Platform?
Without a proper regulatory intelligence tool, regulatory teams are:.

Unable to keep up with the ever-changing regulations and guidelines globally.

Spending too much time researching regulatory requirements for different countries.

Frequently receiving conflicting information from regional partners.

Missing opportunities to engage in industry feedback on draft regulations or guidance.

Constantly reacting to new regulations without sufficient notice and unable to gather the necessary information in time.
