Work smarter with RegDesk’s AI-powered solution for GSPR, ER Checklists, and DoC
Leverage The Power of AI
The number of forms required to show compliance can be overwhelming, and many global agencies still require manufacturers to complete forms separately despite overlapping information. This laborious and repetitive manual process often overwhelms teams.
With RegDesk, you can leverage AI to auto-generate forms and checklists accurately and efficiently. Whether it is GSPR checklists, ER checklists, or the Declaration of Conformity, you can automate it with RegDesk’s AI Form Builder.
This capability of RegDesk is a blessing. We are able to generate GSPRs in a fraction of the time. Additionally, the system automatically manages the impact of changes to standards on our GSPRs and DoCs.
Brian L., Sr. Regulatory Affairs Associate,
Medium MedTech
With RegDesk's AI Form Builder, You Can:
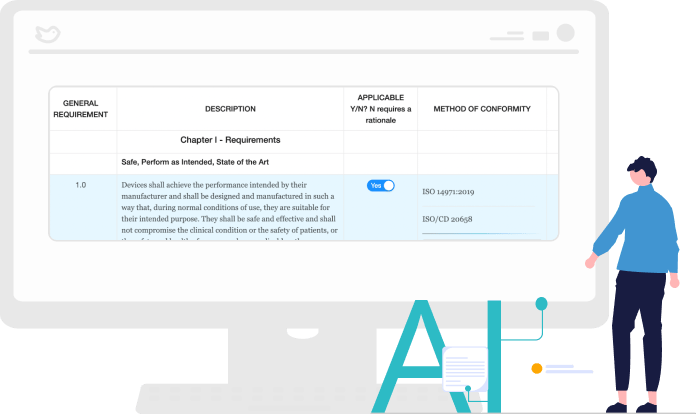
Populate GSPR, ER, and DoCs Using AI Technology
Our AI Form builder for GSPR for medical devices and ER checklists leverages the data input from our Standards Module to fill the forms you need. Our AI learns the content and auto-populates subsequent checklists and DoC forms in an instant.
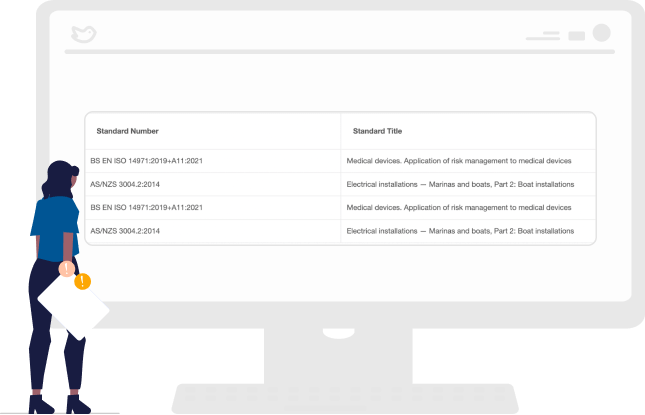
Maintain and Keep the Forms Current
Automatically alerts your regulatory teams of changes to applicable standards. It further specifies which GSPR, ER, and DoCs would be impacted by the change. This automated maintenance keeps forms current so your team doesn’t have to manage them manually across hundreds of checklists.
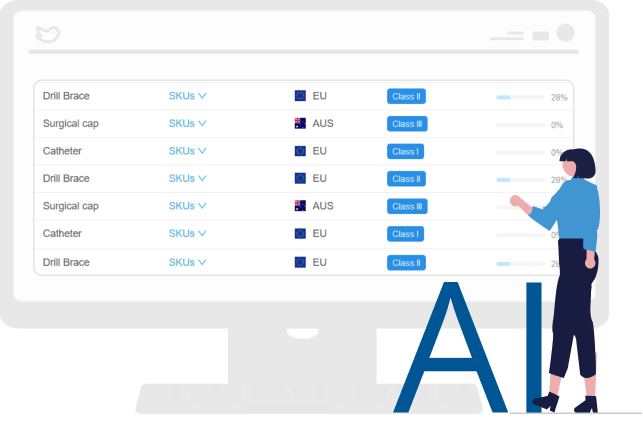
Eliminates human errors
The digitization and automation of the process eliminate potential human errors. It also creates higher quality GSPR, ER, and DoCs with fewer resources, lower costs, and reduced time.
Eliminates human errors
The digitization and automation of the process eliminate potential human errors. It also creates higher quality GSPR, ER, and DoCs with fewer resources, lower costs, and reduced time.

Start Working Smarter Today
It’s never too late to begin streamlining processes and utilizing the power of AI to bring your medical device to market. See for yourself how filling out GSPR for medical devices, ER, and DoCs can be a breeze with an AI-powered form builder from RegDesk.
Can an AI GSPR Form Builder Help Your Team?
Regulatory teams should consider an alternative way to populate these forms if they are:

Spending weeks compiling GSPR checklists , ER, and DoC Forms.

Finding it challenging to integrate content from the Design History Files (DHFs) into the GSPR and ER.

Keeping the forms current and in compliance with standards.

Struggling to ensure the appropriate references to standards are listed in both the GSPR and DoCs.

Dealing with a redundancy of effort and filling in the same information multiple times.
Redundancy doesn’t have to be the reality for your regulatory team. The right AI-powered solution makes it easy to auto-populate these essential forms to streamline the application process.
