What is a PSUR in medical devices?
PSUR is an acronym for Periodic Safety Update Reports. In the medical device context a PSUR is a comprehensive report that provides an evaluation of the safety and performance of a medical device or in vitro diagnostic product over a defined period of time. The report summarizes the manufacturer’s post-market surveillance activities, including adverse event reporting, complaint handling, and other sources of safety information. The purpose of the report is to identify any potential safety concerns associated with the use of the product and to assess the risk-benefit balance.
What information is included in a PSUR?
PSURs typically include the following information:
- Executive Summary: This section provides a brief overview of the report’s content and findings.
- Introduction: This section provides background information on the product, including its intended use, regulatory status, and any significant changes since the previous PSUR.
- Pharmacovigilance Activities: This section describes the manufacturer’s post-market surveillance activities, including adverse event reporting, complaint handling, and other sources of safety information.
- Safety Evaluation: This section summarizes the safety data collected during the reporting period, including any adverse events, trends, and emerging safety concerns.
- Benefit-Risk Assessment: This section evaluates the product’s risk-benefit balance based on the safety and effectiveness data collected during the reporting period.
- Conclusion and Recommendations: This section provides a summary of the report’s findings and any recommendations for further action.
Regulatory Requirements for PSURs:
The regulatory requirements for PSURs vary depending on the jurisdiction and the type of product.
PSUR Requirements EU MDR
In the European Union (EU), PSURs are required for all medical devices and in vitro diagnostic products that have received a CE mark. The frequency of PSUR submission depends on the classification of the product, with higher-risk products requiring more frequent reporting.
PSUR Requirements in the US
In the United States, PSURs are not required by the Food and Drug Administration (FDA) for medical devices. There are alos no PSUR requirements for IVDR. However, manufacturers are still required to conduct post-market surveillance activities and report adverse events to the FDA.
Determine if Your Medical Device or IVDR needs PSUR
If you’re unsure if your device or IVDR needs to go through the Periodic Safety Update Report process, you’re not alone. This diagram should help you understand the path to take.
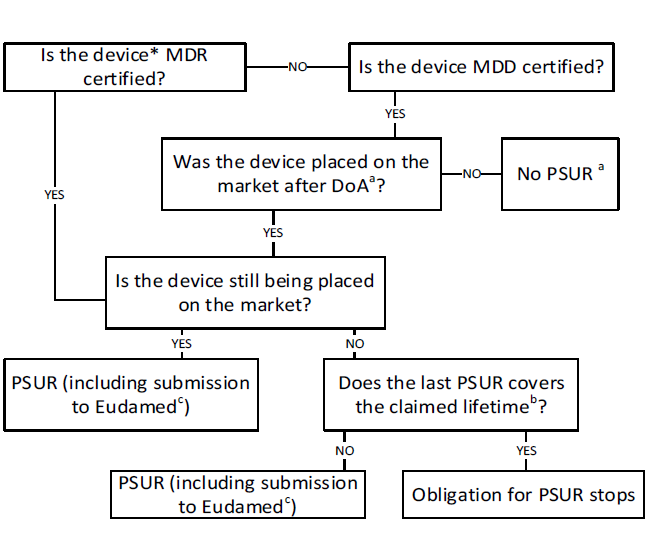
Process for Preparing and Submitting PSURs:
The process for preparing and submitting PSURs involves several steps, including:
- Data Collection: The manufacturer collects safety and performance data for the reporting period, including adverse events, complaint handling, and other sources of safety information.
- Data Analysis: The manufacturer analyses the data to identify any safety concerns or emerging trends.
- Report Writing: The manufacturer prepares the PSUR, including an executive summary, introduction, safety evaluation, benefit-risk assessment, and conclusions.
- Internal Review: The manufacturer conducts an internal review of the PSUR to ensure its accuracy and completeness.
- Submission: The manufacturer submits the PSUR to the regulatory authority or notified body, as required.
Workflow for assessment of PSUR requirement:
To ensure the accuracy and completeness of PSURs, manufacturers should follow some best practices, including:
- Establishing a robust post-market surveillance program that includes the collection and analysis of safety and performance data.
- Conducting regular internal reviews of safety data to identify emerging safety concerns.
- Ensuring that all adverse events and complaints are reported in a timely manner to the regulatory authority or notified body.
- Designing PSURs to be comprehensive and transparent, with clear and concise
- Involving cross-functional teams in the preparation of PSURs, including representatives from regulatory affairs, clinical development, and quality assurance.
- Providing a clear and detailed description of the product and its intended use, as well as any significant changes since the previous PSUR.
- Ensuring that the safety evaluation and benefit-risk assessment sections are based on robust data and analysis and that any limitations or uncertainties are clearly communicated.
- Providing a comprehensive summary of adverse events and other safety data, including a description of the severity, frequency, and outcome of each event.
- Including a thorough review of the product’s labeling and instructions for use, to ensure that they are accurate and up to date.
- Continuously monitoring the safety and performance of the product and updating the PSUR as needed.
The Difference Between a PSUR and PMSR
While both the Periodic Safety Update Report (PSUR) and the Post-Market Surveillance Report (PMSR) serve to monitor the ongoing safety and performance of medical devices, they apply to different device classes and have distinct reporting requirements under the EU MDR and IVDR.
A PSUR is required for higher-risk devices—specifically Class IIa, IIb, and III medical devices and Class C and D IVDs, and must be updated and submitted to the Notified Body (and in some cases via EUDAMED) at defined intervals. It provides a comprehensive evaluation of the device’s benefit-risk profile, sales data, and conclusions from post-market surveillance activities.
The PMSR applies to lower-risk devices (Class I medical devices and Class A/B IVDs) and is generally maintained internally by the manufacturer without formal submission. The PMSR summarizes post-market findings but does not require the same depth of analysis or periodic submission as a PSUR.
How Can RegDesk Help?
RegDesk is an industry leader in Regulatory Compliance in the medical device and IVDR space. Our RIMS platform is designed to help medical device manufactures maintain compliance globally. With regulatory intelligence covering 120+ markets, our centralized platform will help you prepare and publish global submissions, manage standards, conduct impact assessments, and stay ahead of regulatory changes. Expanding into new markets has never been easier. Curious? Reach out today and we’re happy to give you a demo.
 United States
United States 




