Stay Ahead of Global Regulations with Automated Regulatory Intelligence Software
Get a DemoRegDesk integrates regulatory intelligence into the software to keep you up-to-date and compliant
Medical Device and In Vitro Diagnostic companies are able to stay ahead of evolving global regulations and seamlessly integrate into our Regulatory Information Management (RIM) system. Eliminate manual errors and spreadsheets and automate regulatory intelligence to ensure accuracy. With a single source of truth, accelerate market approvals and reduce compliance risks associated with outdated or inconsistent information.
Real-Time Regulatory Updates to Stay Compliant Across Markets
Receive real-time regulatory updates to global regulatory changes to ensure your company remains compliant across multiple markets
Eliminate the burden of manual tracking, reduce compliance risks, and accelerate decision-making processes with region-specific insights and proactive notifications. Our expanded intelligence includes country-specific insight into chemical and substance regulations including RoHS and REACH requirements. Your team will confidently navigate evolving regulations, streamline submissions, and have enhanced compliance management without the need to monitor countless sources of information. With so many updates, it is increasingly important to stay ahead of the ever-changing regulatory landscape.
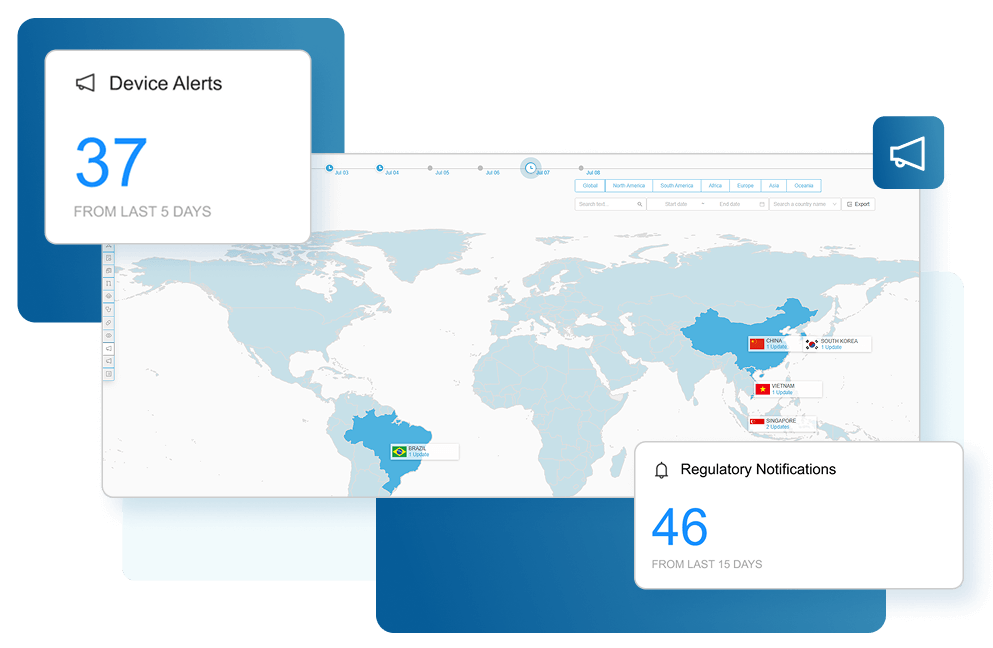
Proactively Manage Regulatory Risk with our Advanced Algorithms
Regulatory Risk Management allows medical device manufacturers to stay ahead of fluctuating regulations. Our team of experts constantly monitors global changes to provide daily alerts, allowing teams to avoid costly delays, quickly pivot strategies, and mitigate compliance risks. To keep devices compliant, proactive notifications and real-time intelligence reduce uncertainty, streamline approvals, and ensure regulatory teams make informed decisions.
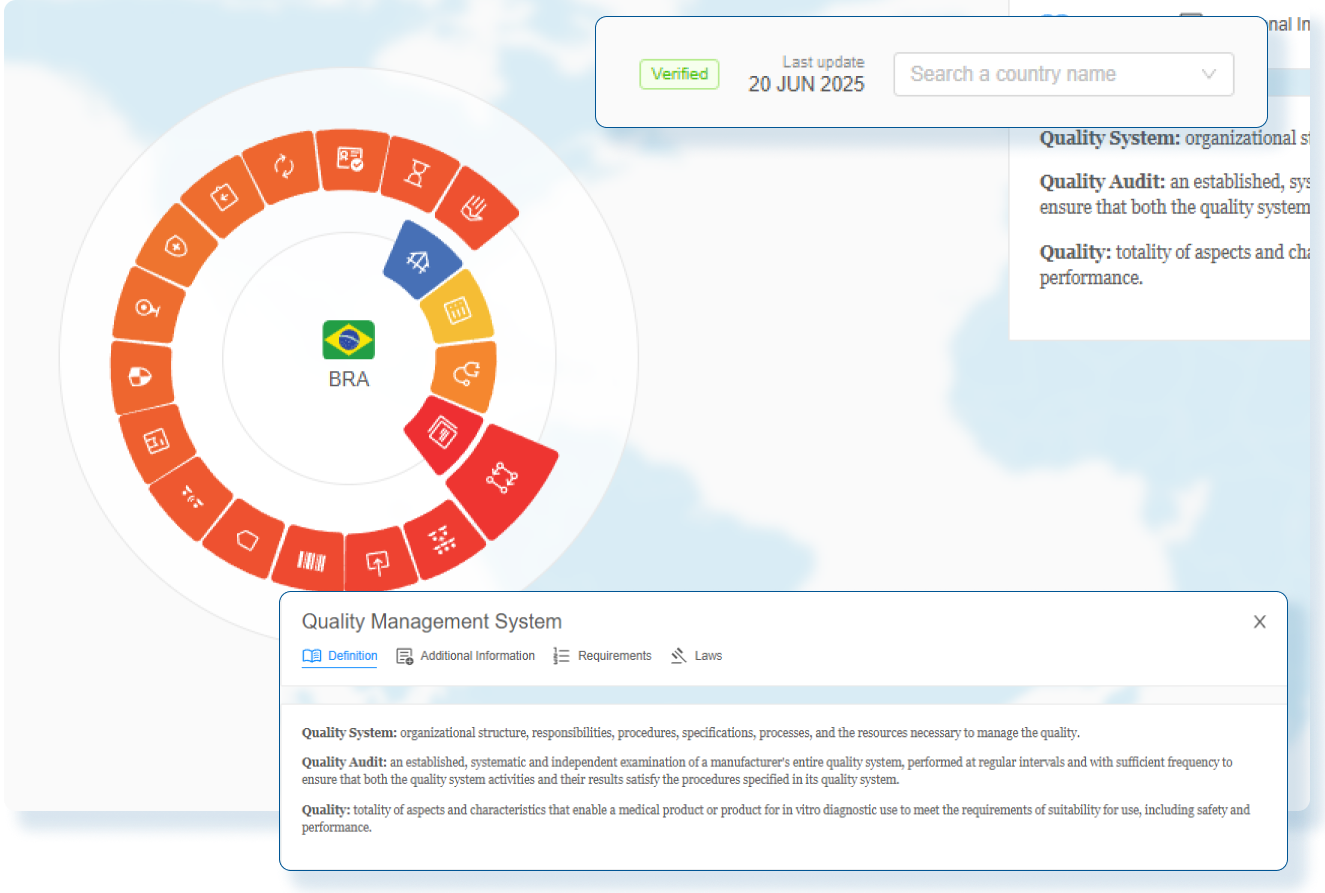
Accelerate Market Access with Real-Time Submission Visibility
RegDesk provides regulatory teams with complete visibility into regulatory submissions and approval statuses, eliminating guesswork and delays. By streamlining workflows, RegDesk accelerates the journey of bringing medical devices to market. With real-time tracking, automated updates, and centralized data, teams can effortlessly stay on top, proactively resolve issues, and speed up the approval process.
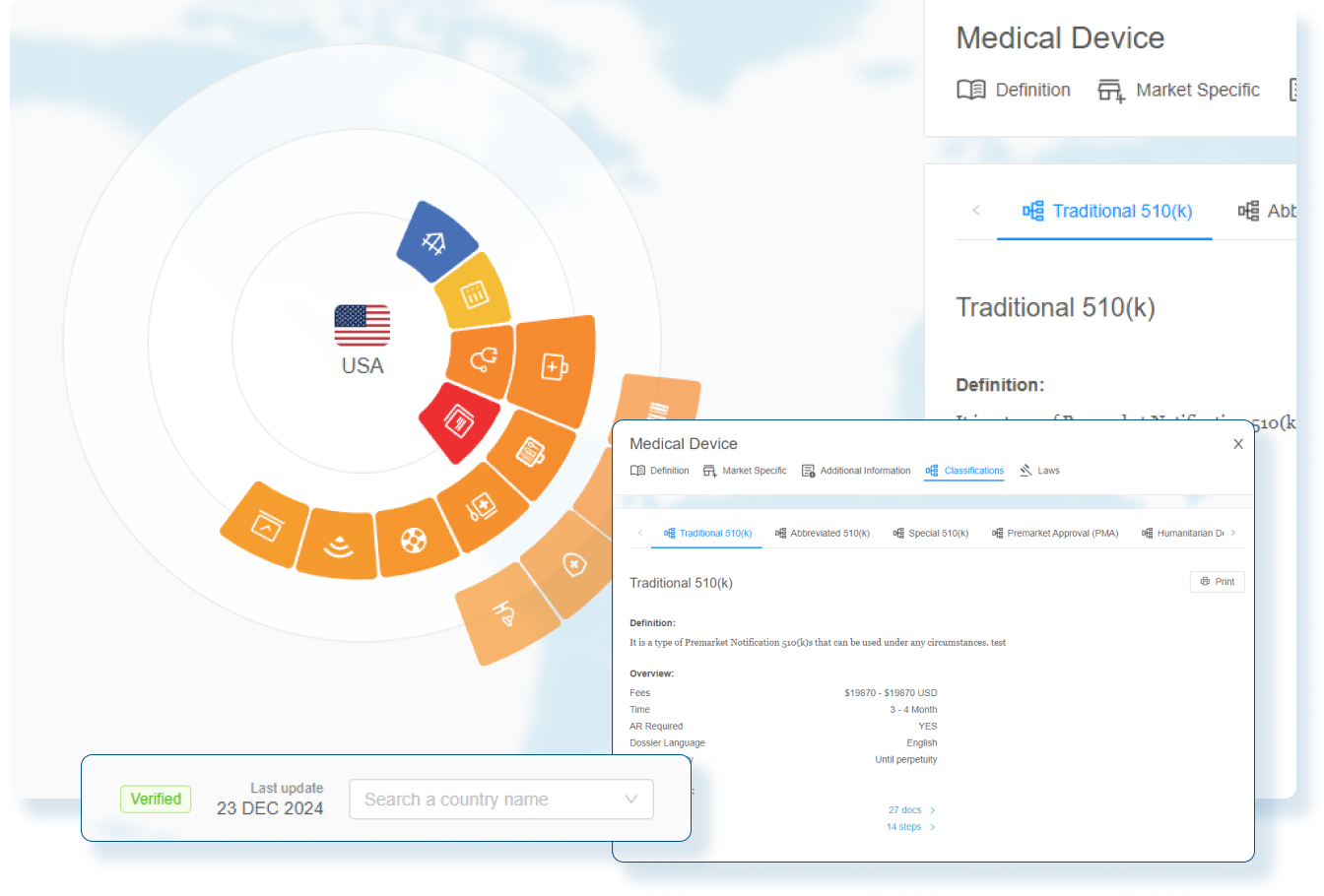
Power Smarter Regulatory Strategies with Real-Time Intelligence
RegDesk’s Regulatory Intelligence Software equips teams with the information to craft a proactive regulatory plan by delivering real-time insights into evolving global requirements.
Powered by AI-driven intelligence, teams anticipate regulatory shifts and make informed decisions that streamline the approval process.
By seamlessly integrating regulatory intelligence into strategic planning, companies can minimize risks, optimize compliance, and confidently support market expansion which ensures a smooth and efficient pathway for device approvals worldwide.
Centralize Regulatory Data to Streamline Compliance
RegDesk revolutionizes regulatory data management by centralizing information, breaking down silos, and fostering seamless collaboration across teams. With automated updates and real-time tracking, compliance efforts become more efficient. Help your team work smarter, streamline submissions, and cut down on manual effort.
“RegDesk has transformed how we manage regulatory data – everything is in one place, up to date, and accessible across teams.”
Take Charge of Your Regulatory Strategy
Experience how our RIMS platform integrates into your framework to streamline compliance, speed up approvals, and eliminate manual inefficiency.
Request a DemoFrequently Asked Questions
What is the role of regulatory intelligence in the medical device industry?
Regulatory intelligence plays a crucial role in keeping medical device companies ahead of the curve. By offering real-time insights into the constantly evolving global regulations, it empowers teams to stay compliant and navigate complex approval processes with ease. It enables companies to track regulatory updates, understand market entry requirements, and build sturdy regulatory strategy plans that streamline compliance and accelerate time-to-market, ultimately expediting patient access to medical devices.
How does regulatory intelligence impact the product lifecycle?
Regulatory intelligence plays a pivotal role throughout the entire product lifecycle. When regulatory affairs teams are able to identify key regulatory requirements, optimize submission processes, and ensure ongoing compliance, it minimizes delays and speeds up product approvals. These informed decisions enhance market access, strengthen patient safety, and ensure a smoother path to compliance at every stage.
Who can benefit from having regulatory intelligence?
Regulatory intelligence is a game-changer for regulatory affairs teams, quality assurance professionals, compliance managers, and leadership across medical device and in vitro diagnostics companies. With centralized, AI-powered insights, these teams can make data-driven decisions, stay ahead of regulatory shifts, and ensure compliance across global markets, ultimately responding faster and more effectively to ever-changing requirements.