Streamlined Standards Management
Get a DemoThe obstacles to launching a medical device can be daunting. Compliance with international and country-specific standards is paramount to success. Without a purpose built regulatory platform teams often face costly errors and missed updates that impact your ability to register and maintain approval of a medical device through its lifecycle. RegDesk centralizes standards management, ensuring teams stay organized, audit ready, aligned with regulations, and compliant with minimal risk.
Stay Up-to-date on Changes to Standards
RegDesk simplifies managing medical device standards and regulatory requirements, ensuring compliance with global regulations such as ISO, ASTM, Chinese International Standards (GP), Chinese Industry Standards (YY), and Global Harmonized Standards.
The centralized platform provides real-time alerts on changes to standards, workflows for gap analyses, and streamlined documentation. By integrating regulatory and quality management activities, medical device companies are able to maintain the highest standards of performance and compliance across their entire product portfolio.
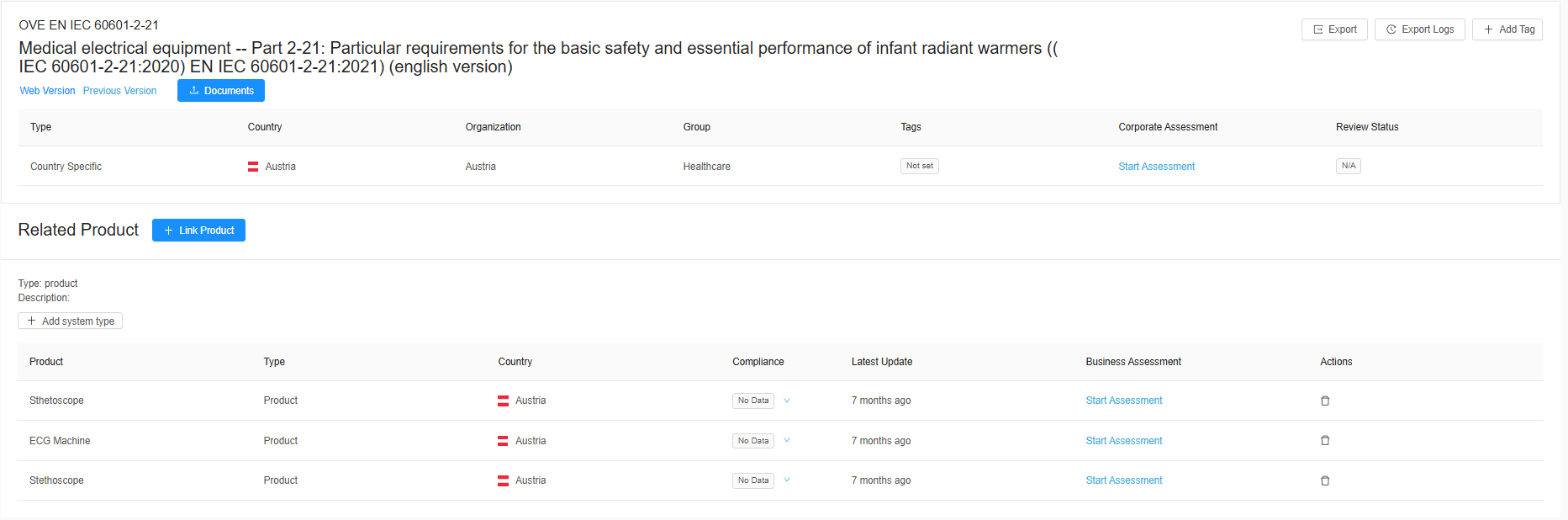
Compliance with Guidances
Regulatory compliance is essential for market access. With an unprecedented amount of updated guidelines, RegDesk’s proactive alerts keep regulatory teams ahead of changes to medical device regulations, including draft guidances, minimizing the risk of non-compliance. By streamlining monitoring, RegDesk ensures that devices consistently meet evolving regulatory requirements across regions.
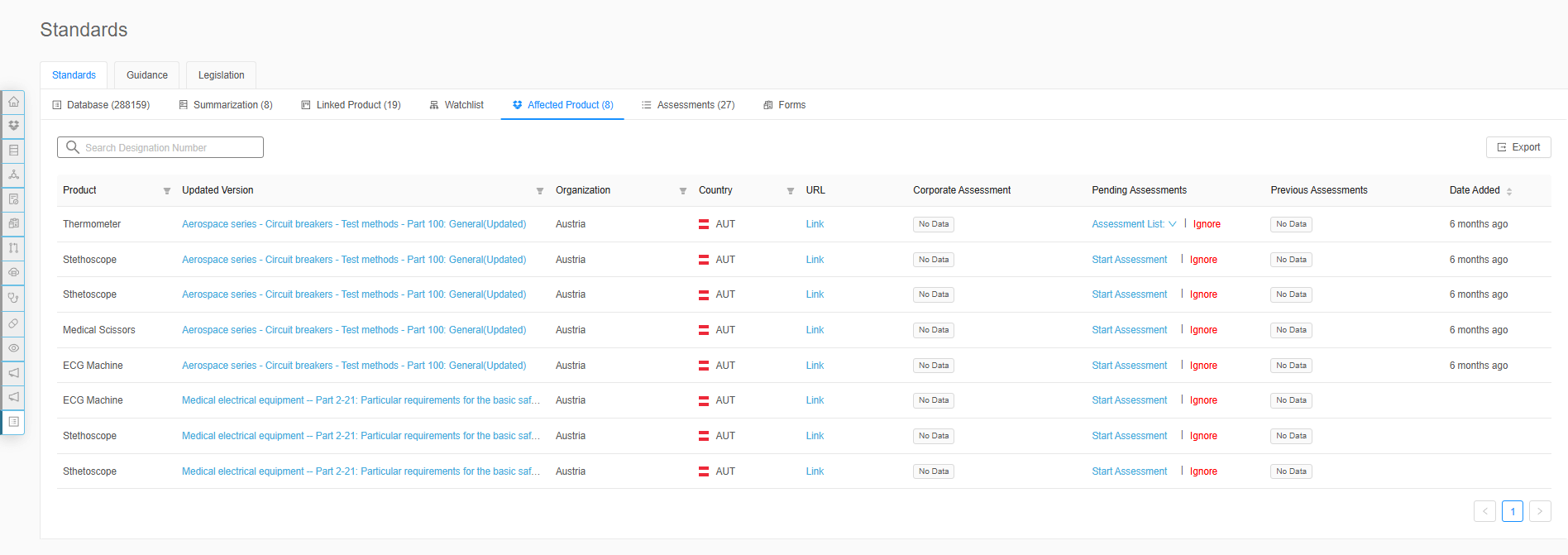
Seamless Implementation Across Teams
Effective implementation of medical device standards management requires collaboration across regulatory affairs, quality assurance, and product engineering teams. To avoid delays in compliance and efficiency, it’s crucial to engage stakeholders early and implement technology that simplifies processes, integrates seamlessly with existing systems, and supports audits. With strategic planning, well defined roles, and continuous training, having a successful integration with RegDesk is simple and easy.
Stop Struggling With Out of Date Information
Stay ahead of changing standards and ensure compliance with RegDesk’s comprehensive Medical Device Standards Management solution.
Get a DemoFrequently Asked Questions
What is medical device standards management?
Medical device standards management is the process of identifying, tracking,
implementing, and maintaining relevant medical device standards as they apply
to the design, development, testing, manufacturing, and post-market surveillance
of medical devices. This ensures that medical devices have been rigorously tested and meet safety requirements across global markets. Tools that centralize and automate this process help with compliance, faster market access, risk reduction, and product safety.
What is ISO 13485 standard for medical devices?
The primary ISO standard for medical devices is ISO 13485. This outlines the specific requirements an organization must meet to demonstrate its ability to consistently meet customer and regulatory requirements. ISO 13485 focuses on risk management, design and development, traceability and documentation, and post-market surveillance, which are applicable throughout the entire product lifecycle.
Are manufacturers required to track all medical devices to the end user?
The requirements for manufacturers can vary depending on device classification and country requirements. To obtain medical device compliance, many countries have regulations that emphasize the need for proper tracking systems to monitor devices throughout their lifecycle. Tracking ensures traceability for safety purposes, particularly in the case of recalls or safety issues.





