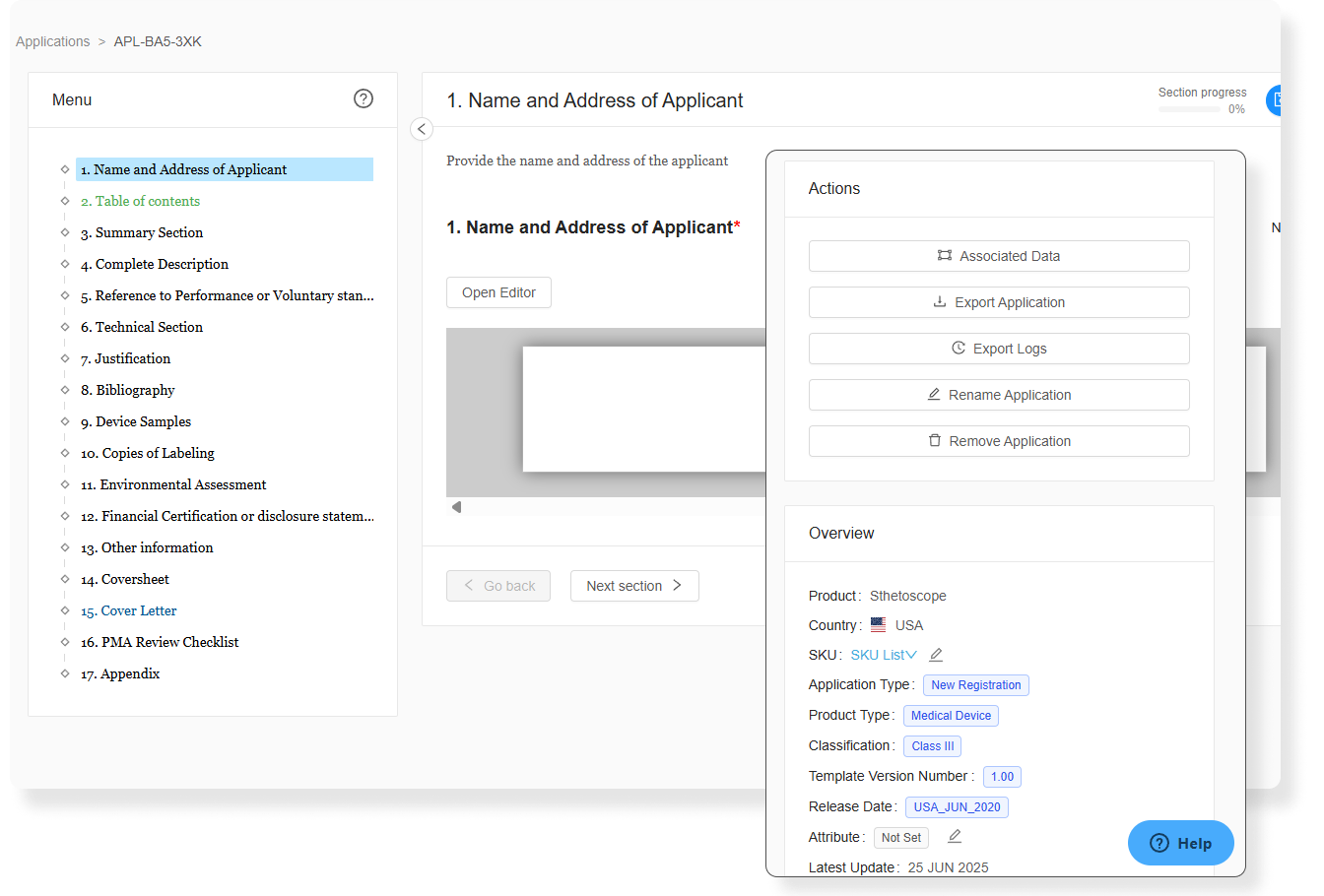
Accelerate Submissions with AI-Driven Automation
AI-Powered Regulatory Submissions, Smarter and Simplified
AI is revolutionizing regulatory affairs, transforming how manufacturers manage global compliance. By automating repetitive tasks like medical device submission preparation, 510 (k) filings, GSPR checklists, and Pre-Market approvals, AI significantly reduces human errors and saves time. With enhanced accuracy and fewer delays, manufacturers can be confident in their regulatory submissions while accelerating time to market without adding headcount.
Local Compliance Made Easy with Country-Specific Templates
RegDesk’s AI utilizes an extensive library of country-specific templates, ensuring every submission follows local formatting, content, and language standards. This not only expedites global expansion but also minimizes the risk of costly rework due to compliance discrepancies.
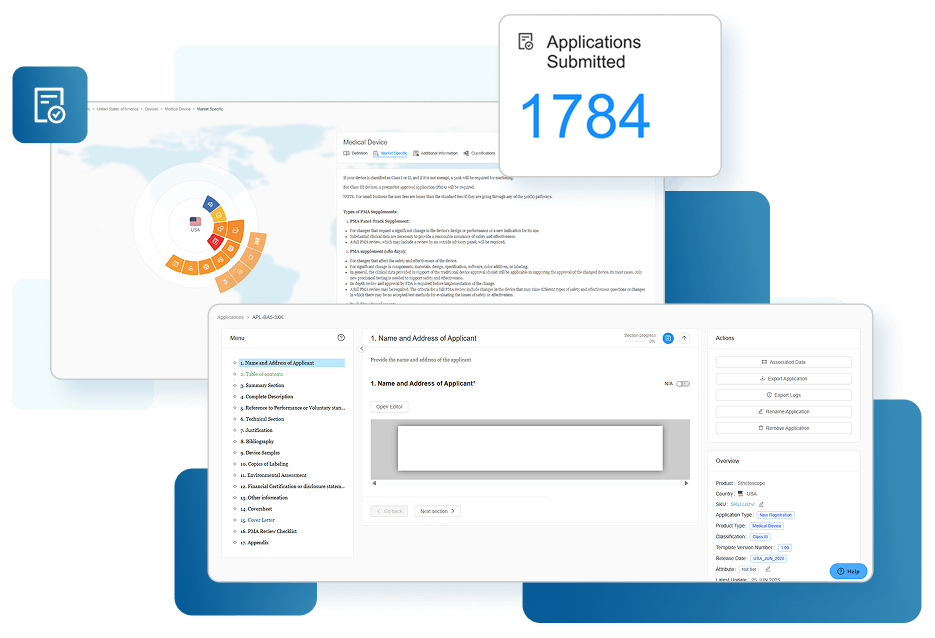
Reduce Submission Time with Smart Autofill
Once product details are uploaded, RegDesk’s AI autofills subsequent applications using previously submitted data. This eliminates duplication, reduces errors, and dramatically cuts down preparation time across multiple markets, making the submission process more timely and efficient.
Unlock the Full Potential of AI in RIMS
Faster Medical Device Submissions
Accelerate the creation of dossiers for EU MDR, 510(k), Pre Market Approval, and other international submissions slashing time-to-market by months.
Minimized Manual Errors
AI ensures data consistency and accuracy across all submissions, reducing the risks associated with human error and compliance issues.
Simplified Country-Specific Compliance
Leverage pre-built templates and rulesets to easily adapt submissions to meet each market’s unique regulatory requirements.
Optimized Resource Allocation
Free regulatory teams from redundant tasks, enabling them to focus on high-impact strategy, planning, and strategic initiatives.
Up-to-date Templates
Stay ahead of global regulatory changes with updated country-specific submission templates.
Automated GSPR Checklists & DoCs
Instantly generate and update GSPR and Essential Principles Checklists, as well as Declaration of Conformity (DoC) forms for seamless EU MDR and global compliance.
Scalable Global Operations
Easily manage submissions across multiple regions using AI to scale operations effectively and intelligently.
Auto-Generated Regulatory Forms in Seconds
See how it worksRegDesk’s AI technology automatically creates the mundane regulatory forms needed for Medical Device Submissions across most regions. By eliminating manual data entry and aligning with local regulatory requirements, our technology simplifies and streamlines the submission process. You can save time, minimize errors and move forward efficiently with confidence.
See how it worksSmarter, Automatically Populated GSPR and Essential Principles Checklists
Our platform provides GSPR (General Safety and Performance Requirements) Checklists customized for specific regulatory frameworks such as the EU MDR, Australia, Saudi FDA, and more. AI automatically populates checklists with relevant data collected from your submission and standards management, ensuring compliance with changing requirements and saving hours of manual input and management.
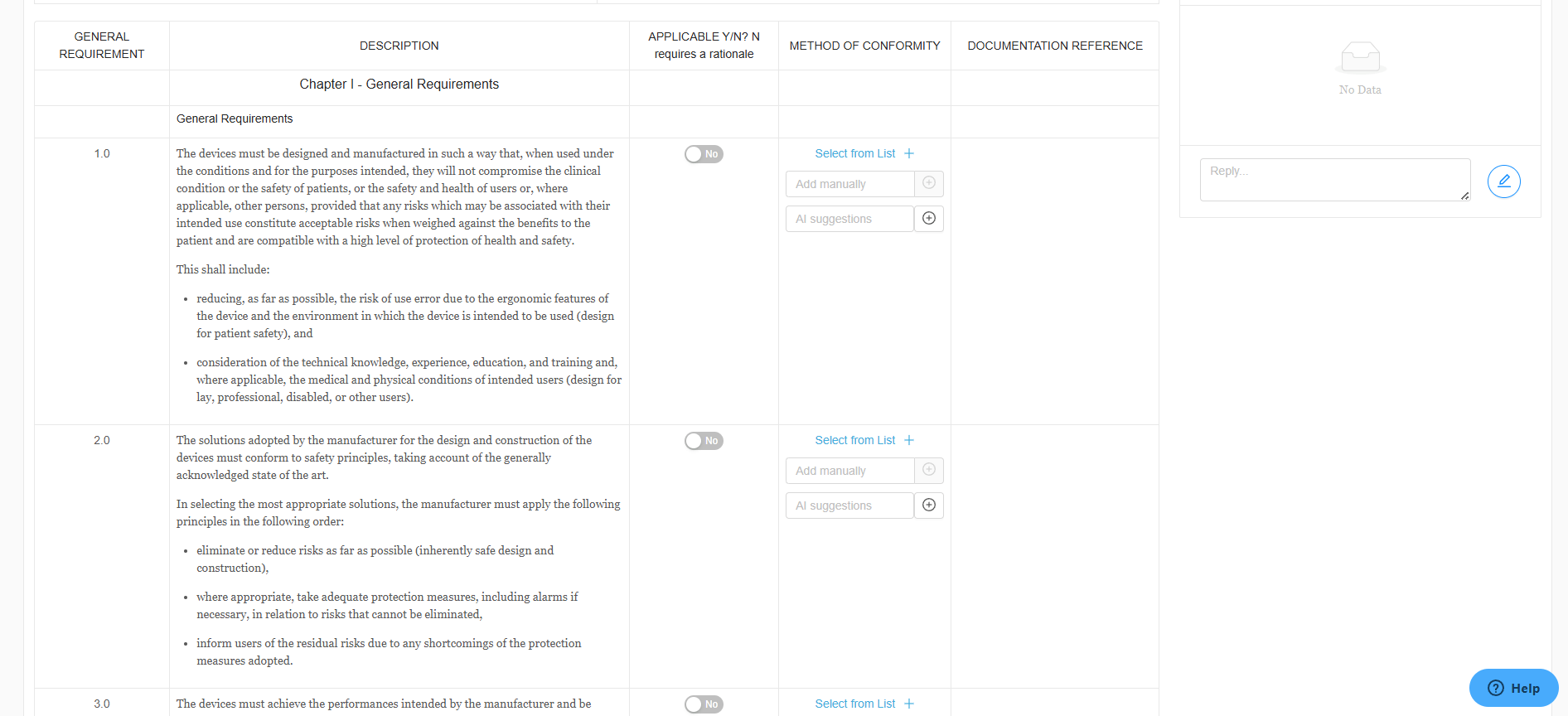
Simplified Essential Requirements Management
RegDesk simplifies the management of Essential Requirements by automatically cross-referencing them with existing standards for each market. Our AI technology detects any gaps and provides timely updates, ensuring your submissions stay aligned with the latest regulations. Save time and money while streamlining your process with accuracy and reliable data.
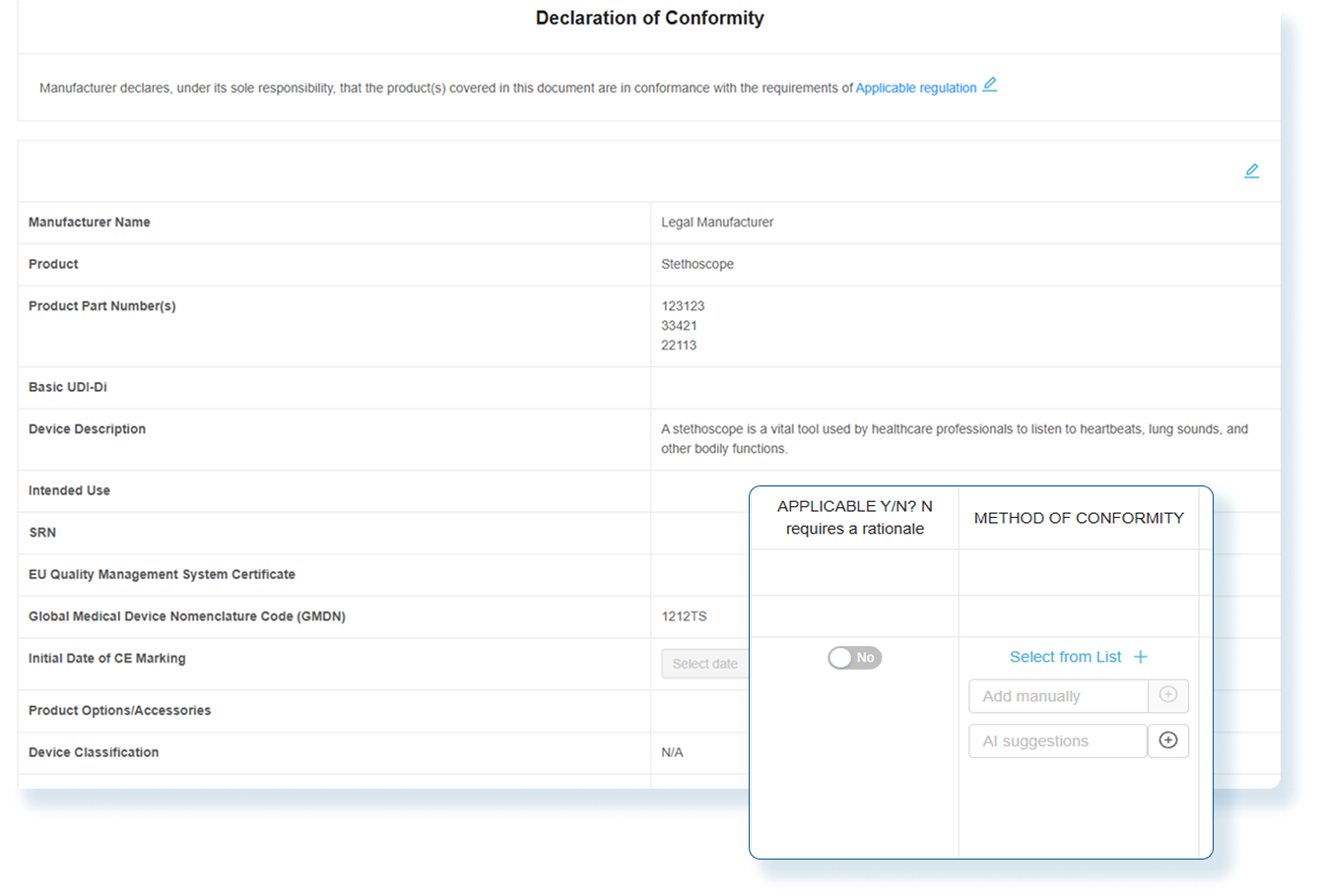
Effortless Declaration of Conformity (DoC)
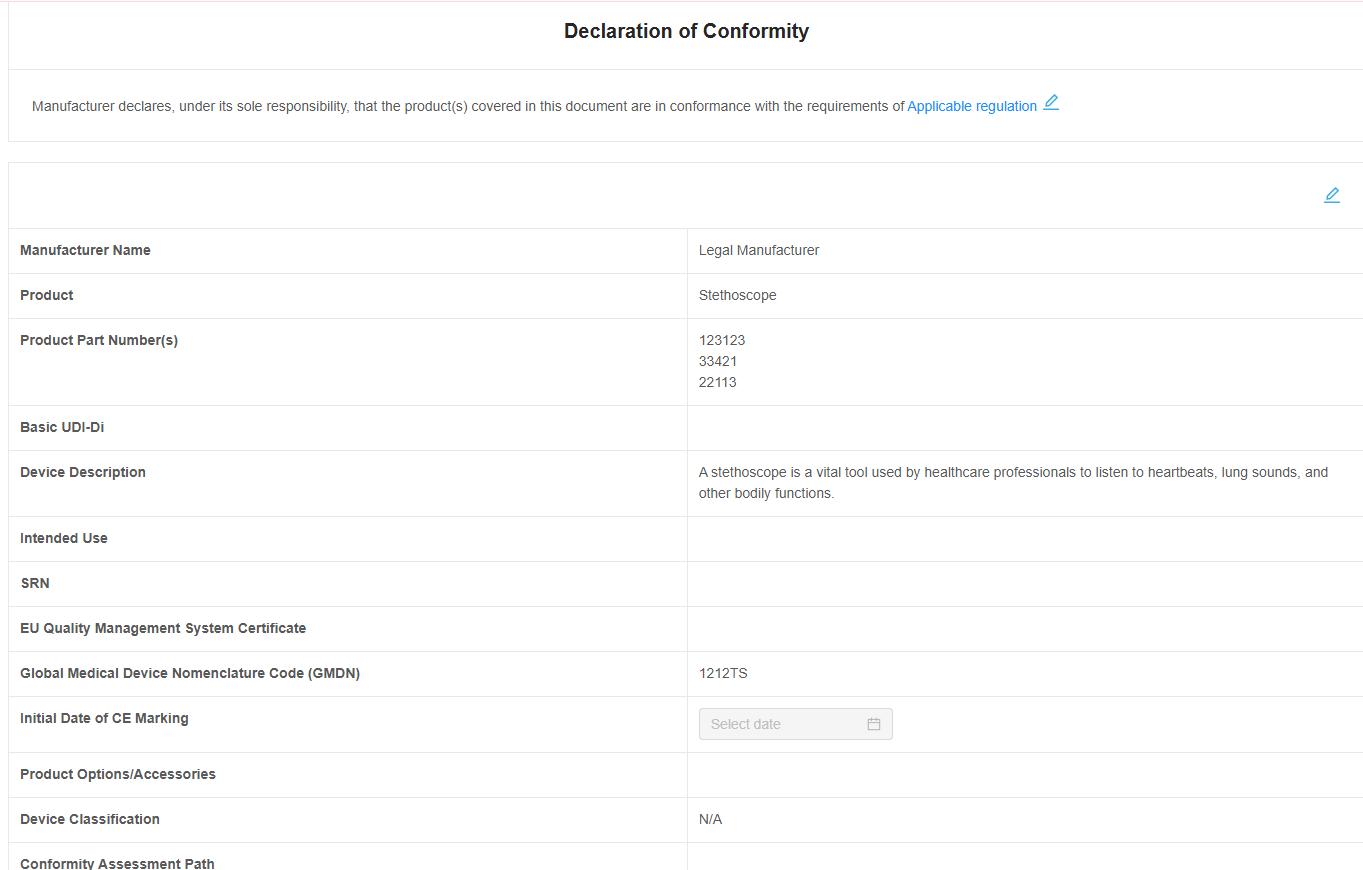
With RegDesk’s assistance, complete the Declaration of Conformity (DoC) forms quickly and accurately. By utilizing our AI technologies, easily generate and fill the required sections, ensuring all important information meets regulatory requirements. Partner with RegDesk and move from submission to approval faster and with ease.
Learn MoreRegulatory Alerts for Ongoing Maintenance and Compliance
Ensure your team is up-to-date with the newest regulatory updates with RegDesk’s Regulatory Alerts
Automatically get updated when changes in standards impact your GSPR, Essential Principles Requirements (EP), and Declaration of Conformity (DoC) forms. Allow RegDesk to make ongoing maintenance an easy box to check off while ensuring compliance remains a top priority.
Revolutionize Your Regulatory Process with
AI-Powered Solutions
Learn how partnering with RegDesk can create market expansion and ensure global compliance.
Get a DemoFrequently Asked Questions
How can AI improve the accuracy of regulatory submissions??
AI enhances the precision of regulatory submissions by automating much of the submission authoring process while ensuring compliance with requirements. It also reduces human error by cross-referencing data with regulatory standards and templates, guaranteeing that all necessary information is accurate, complete, and consistent across every document.
How can AI help medical device companies stay ahead of regulatory changes?
AI enables medical device companies to stay ahead of regulatory changes by monitoring global regulatory updates and automatically alerting teams to relevant changes. It tracks evolving requirements and seamlessly incorporates them into the submission process, without the need for manual effort.
How is AI used in regulatory compliance?
AI is transforming regulatory compliance by automating tasks such as generating submission documents, autopopulating compliance checklists and standards forms, and managing regulatory alerts. It can process large amounts of regulatory data, predict potential compliance risks, and ensure alignment with global standards. This not only boosts efficiency but also significantly reduces the risk of non-compliance.