RegDesk Regulatory Compliance
Software For Medical Devices
Regulatory Compliance Simplified
The only RIM platform, with built-in regulatory intelligence and AI-powered automation that allows management of change assessment and preparation of international submissions within hours compared to months.
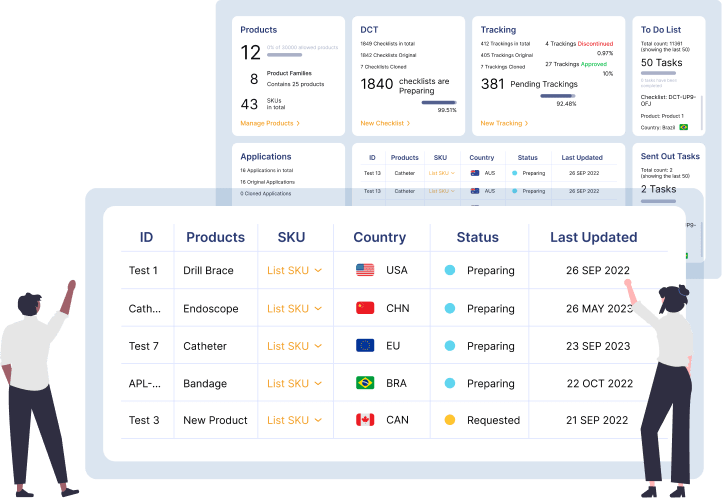
Dashboard to track global registrations
What is RegDesk?
Complying with differing global regulations creates exponential complexity – and the pace of change continues to accelerate. To keep pace, investing in a holistic regulatory platform has become critical. RegDesk can help.
TRUSTED BY LEADING COMPANIES
BENEFITS OF REGDESK'S RIM PLATFORM
Prepare international applications within 1 week instead of 4 months
Reduce response time to address international inquiries from 6-8 weeks to less than 20 hours
Avoid stress and nonconformities from a Health Agency audit
Why RegDesk RIMS
With RegDesk, expand to 120+ global markets ahead of your competitors. We make compliance with global and local laws and regulations easy for specialized pharma and medical device companies.
This is what makes us unique
Regulatory Intelligence
Develop fast and reliable regulatory strategy and manage potential vulnerabilities proactively through our comprehensive regulatory intelligence.
- Access up-to-date regulatory requirements for 120 markets
- Receive daily alerts on changing regulations
- Obtain timelines and costs per country
AI Submission Generator
Reduce your time to prepare, publish, and submit global applications from months to only a week using RegDesk’s AI technology.
- Access country-specific, submission ready templates
- Prepare and publish global applications in formats acceptable by regional health agencies
- Leverage AI to autopopulate applications for subsequent countries
Tracking & Reporting
Monitor the entire lifecycle of your product and never again miss a renewal or ship a wrong product to a country.
- Track the status of registrations by product name and SKU
- Receive renewal notifications
- Generate reports on key performance indicators within seconds
Standards Management
Maintain compliance of standards and guidances worldwide and avoid non-conformities from audits.
- Search for international and country-specific standards and guidance documents
- Manage gap assessments on standards through integrated workflows
- Receive notifications on changing standards and guidance documents that affect your products
Change Assessment
Manage global impact of changes to a product faster and with ease through the Only Change Assessment Tool available with built-in regulatory intelligence.
- Understand the regulatory impact of changes to an existing product
- Generate report to see which products, countries, and SKUs will be impacted
- Manage change control workflows to collaborate with internal and external teams
AI Form Builder (GSPR, DoC, Requirements)
Decrease staff hours and errors by auto-generating GSPR, Declaration of Conformity (DoC), and Essential Requirements documents using RegDesk’s AI capability.
- Utilize AI and machine learning to auto-populate GSPR, DoC, and Essential Requirements
- Track which submission documents require change when a standard changes
- Automate bulk updates of the GSPR, DoC, and Essential requirements
Testimonials
Industries We Serve
Medical Device
Holistic RIM Solution with built-in Regulatory Intelligence for:
- Medical Devices
- In Vitro Diagnostics
- Software as a Medical Device
- Radiation Emitting

Pharmaceutical
Comprehensive Regulatory Intelligence for:
- Pharmaceuticals
- Biologics
- APIs
- Excipients
Сountries we serve
We can help you expand to 120+ markets in the world.
REGDESK REGULATORY ROUNDUP
Never miss a thing in the world of medical device regulatory compliance. The latest regulatory news and updates are always on our feed.





















